A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system


Опубликована Янв. 15, 2013
Последнее обновление статьи Дек. 26, 2022
Abstract
Ginseng is one of the most widely used herbal medicines in human. Central nervous system (CNS) diseases are most widely investigated diseases among all others in respect to the ginseng's therapeutic effects. These include Alzheimer's disease, Parkinson's disease, cerebral ischemia, depression, and many other neurological disorders including neurodevelopmental disorders. Not only the various types of diseases but also the diverse array of target pathways or molecules ginseng exerts its effect on. These range, for example, from neuroprotection to the regulation of synaptic plasticity and from regulation of neuroinflammatory processes to the regulation of neurotransmitter release, too many to mention. In general, ginseng and even a single compound of ginsenoside produce its effects on multiple sites of action, which make it an ideal candidate to develop multi-target drugs. This is most important in CNS diseases where multiple of etiological and pathological targets working together to regulate the final pathophysiology of diseases. In this review, we tried to provide comprehensive information on the pharmacological and therapeutic effects of ginseng and ginsenosides on neurodegenerative and other neurological diseases. Side by side comparison of the therapeutic effects in various neurological disorders may widen our understanding of the therapeutic potential of ginseng in CNS diseases and the possibility to develop not only symptomatic drugs but also disease modifying reagents based on ginseng.
Ключевые слова
Alzheimer's disease, Ischemia, Panax ginseng, Parkinson's disease, Neurodevelopmental disorders
INTRODUCTION
Ginseng is any one of tire perennial plants, which are included in genus Panax and family Aralliaceae. Among eleven different species of ginseng commonly called as ginseng, three species of ginseng, i.e., Panax ginseng (commonly called as ginseng or Korean ginseng), P. quinquefolius (commonly called as American ginseng) and P. notoginseng (commonly called as Chinese no- toginseng or Sanchi) are the three most commonly used ginseng herbs at present [1]. Ginsenoside is believed to be the active compounds of ginseng herbs and widely used for the pharmacological examination of effects of ginseng ranging from the role as a traditional nourishing stimulant to anticancer reagent. Ginsenosides are classified into three categories based on their structural differences. The panaxadiol group includes Rbl, Rb2, Rb3, Re, Rd, Rg3, Rh2, and Rsl and panaxatriol group includes Re, Rf, Rgl, Rg2, and Rhl. Ro is classified as oleanolic acid group [2]. The investigation on die beneficial and sometimes harmful effects of ginseng in various pathological conditions keeps growing to previously unexpected extents and for example, it has been suggested that the combination of red ginseng with highly active anti-viral therapy may improve die clinical outcome of human immunodeficiency virus type 1-infected patients[3]. Growing number of publications are also attributed to tire effects of ginseng and ginsenosides on central nervous system (CNS) disorders. These disorders in CNS are not only confined to the traditional target diseases such as stress and ischemia but also expand into more recently acknowledged neurological and psychiatric disorders including Alzheimer's disease (AD) and attention deficit hyperactivity disorder (ADHD).
Recently, several reviews are published about the role of ginseng or ginsenosides on neurological functions or possible target proteins including different types of ion channels. For example, ginseng or ginsenoside affects nemotransmission of acetyl choline and gamma- aminobutyric acid (GABA) by mechanism involving the regulation of the expression of synthetic enzyme, neurotransmitter release as well as the signaling pathways involved in tire particular nemotransmitter systems. Ginseng and ginsenosides improve learning and memory as determined by behavioral analysis by mechanism involving alteration of synaptic plasticity and increase in neurogenesis, thereby affecting neuronal density in hippocampus. Ginsenosides affect voltage and ligandgated ion channels including K+, Na+, and Ca2+ channels as well as N-methyl-D-aspartate (NMDA)-, nicotine-, and serotonin-gated ion channels (for a review, see [4]). Recently, growing expectations about tire role of ginseng and ginsenosides in the regulation of stem cell proliferation and differentiation as well as the more comprehensive understanding of classical neurotrophic role of ginseng may confer enthusiasm in investigating the role of ginseng in CNS disorders such as stroke, AD, Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington's disease (HD). At tire moment, the most extensively studied effects of ginseng and related compounds me their effects on stroke and AD. In addition to tire descriptive studies depicting the efficacy of ginseng and ginsenosides in annual models of disease as well as their nemoprotective role in terms of anti-oxidant, antiinflammatory, and anti-apoptotic activity, more specific cellular and moleculm mechanisms me now revealed, which dictates close overviews on the role of ginseng and ginsenosides in CNS disorders. However, extensive reviews on the role of ginseng on fire neurological and psychiatric diseases me scarce not to mention fire mechanism of ginseng mediating fire regulatory role in those diseases. In this review, we will summarize the works conducted regarding the effects of ginseng (fire three conventional ginseng species excluding Siberian or Indian ginseng) and ginsenosides in various CNS diseases using cell culture and annual models as well as human clinical studies in an hope to systematically understand the effects and mechanism of ginseng in CNS disorders.
ALZHEIMER'S DISEASE
With the in-depth understanding of moleculm and cellular pathophysiology and neurobiology of AD during last couple of decades, many plausible targets to treat AD have been suggested which include but not restricted to followings: 1) increase in fire uptake of choline in central nervous system, 2) release of acetylcholine from hippocampus, 3) increased activity or expression of choline acetyltransferase, 4) protection against the Aß or tan protein-induced neurotoxic effects by several mechanisms including inhibition of neuroinflammation, increased production of neurotrophic factor, and regulation of apoptotic processes, 5) repair of Aß-dmnaged neuronal networks by increased nemogenesis and synaptic plasticity, and 6) reducing fire level of Aß by decreased production or increased elimination. Actually, many of these pathophyiological targets and tire effects of ginseng and ginsenosides on them me subjected to tire intense investigation and covered by several recent reviews [5,6].
Regulation of neurite outgrowth and synaptic plasticity by ginseng
Based on tire fact that protopanaxadiol-type saponins were active constituents mediating neurite outgrowth in human nemoblastoma SK-N-SH [7], it has been demonstrated that ginsenoside Rbl and its intestinal metabolic compounds Ml recovered the unpaired spatial memory and expression levels of phosphorylated NF-H and syn- aptophysin in an annual model of AD induced by intra- cerebroventriculm injection of Aß(25-35) [8]. In cultmed cortical neurons, Ml increased axonal outgrowth even after substantial progress of neurite degeneration, suggesting a nemo-regenerative potential of tins compound. Similar neurite outgrowth potential of crude ginseng saponin was also reported in cultmed rat cerebral cortical neurons [9]. In addition, ginsenoside Rbl potentiated the nerve growth factor (NGF)-mediated neurite outgrowth of cultured chick embryonic dorsal root ganglia [10]. Interestingly, the memory impairment induced by intracerebroventriculm injection of Aß(25-35) as well as synaptic marker protein expression is only marginally increased by donepezil, if any, consistent with its primary mechanism of action, i.e., tire inhibition of acetylcholine esterase [8]. In addition, tire memory improving effects of Rbl or Ml were maintained even after the discontinuation of Rbl and Ml administration suggesting they induced a long lasting and probably structural reorganization of tire damaged brain circuits, which is ideal for tire treatment of neurodegenerative conditions including AD although further verification in oilier annual models of AD is needed in tire future. Neurite outgrowth is one of the foremost phenotypical changes in network reorganization, which might be one of the cellular and molecular changes happening during synaptic plasticity, which suggests that the observed neurite extension effects of ginseng may underlie the memory enhancing effects of ginseng in both normal and pathological conditions, although clinical studies produced both positive and negative results (see below).
In tire dentate gyrus of anesthetized rats, i.c.v. injection of ginsenoside Rbl (10, 100 nM) inhibited the induction phase of long term potentiation (LTP) and accelerated tire maintenance phase of LTP induced by high frequency stimulation in a dose-dependent manner [11]. In addition, treatment with nonsaponin fraction of ginseng significantly ameliorated deficits in place-navigation learning in tire aged rats in the place learning task along with significant augmentation in the increase in population spike amplitudes in the CA3 subfield after LTP induction in vilro [12]. Similarly, ginsenoside Rgl inhibited morphine induced spatial memory deficit which was determined by Morris water maze test and restored LTP impaired by morphine in both freely moving and anaesthetized rats, which was examined by electrophysiological recording after implantation of electrodes in vivo [13]. The electrophysiological recording in vitro also demonstrated that Rgl restored the LTP in slices from the rats treated with morphine without affecting LTP in the slices from normal rats. The restoration LTP is inhibited by NMDA receptor antagonist MK801 suggesting the involvement of NMDA receptor activation in the Rgl-induced recovery of LTP [13]. Using acutely isolated rat hippocampal CA3 pyramidal neurons with a conventional whole-cell patchclamp teclmique, it has been suggested that Compound K, a metabolite of Rbl, enhances spontaneous GABA release by increasing intraterminal Ca2+ concentration via Ca2+ release from pre-synaptic Ca2+ stores, although how tliese findings are related to the regulation of hippocampal excitability and learning and memory processes remains to be determined [14].
Morphologically, Rgl increased chronic mild stress induced decrease in dendritic spine number and hippocampal neurogenesis [15], which also suggests the modulation of processes involved in synaptic plasticity by ginsenosides.
Ginsenoside Rgl and Rblincreased proliferation and differentiation of neural progenitor cells in dentate gyrus of hippocampus of normal adult mice and global ischemia model in gerbils. In addition, Rgl increased expression of brain derived neurotrophic factor, Bcl-2 and antioxidant enzyme and increased the number of synapses and mossy fiber sprouting in CA3 regions of hippocampus suggesting the role of Rgl in the modulation of synaptic plasticity and possibly to the increased cognitive function in AD [16]. Similarly, when the mixture of brain-derived neurotrofic factors (BDNF) and ginsenosides Rgl and Rbl was treated to human neural stem cell during the differentiation procedure, it promoted cell survival and enhanced neurite outgrowth and the expression of synaptic marker proteins, which was evidenced by tune lapse microscopy, immunostaining, and Western blot [17].
Neuroprotection
Ginsenosides have direct neuroprotective effects against glutamate or Aß stimulation. In cultured PC 12 cells, glutamate decreased cell viability and increased intracellular calcium concentration and lipid peroxidation, which is evidenced by the excessive production of malondialdehyde and nitric oxide (NO) [18], all of which are prevented by ginsenosides. Whether die neuroprotective effects against glutamate has been related with die reported antagonistic activity of many ginsenoside such as Rg3 and R112 against NMDA receptors remains to be determined (for a review, see [19]). The antioxidant effects of ginseng has also been implicated in a study showing die neuroprotective effects of ginseng extracts in human neuroblastoma SY-5Y cells [20]. Treatment of SY-5Y cells with cyclosporine A inhibited calcineurin activity, which results in hyperphosphorylation of tan protein. Pretreatment of ginseng extracts effectively enhanced calcineurin activity, which ameliorates tan phosphorylation providing possible neuroprotective activity [20]. Treatment of ginsenoside Rg2 effectively and significantly attenuated the glutamate-induced toxicity and changes in above mentioned factors. In addition, ginsenoside Rg2 decreased the level of glutamate-induced increased protein expression such as calpain II, caspase-3, and Aß(l- 40) in PC12 cells. In addition, treatment of water extracts of American ginseng significantly attenuated the cellular apoptosis of SH5-SY cells induced by Aß(25-35), which was determined by staining with Hoechst 33258 [21]. Treatment of 50 pM Aß(25-35) for 48 h also produced cell toxicity in PC 12 cells and treatment of ginsenoside Rgl inhibited ß-secretase activity in vitro and protected PC12 cells from cell death along with inhibition of NO release, lipid peroxidation, reactive oxygen species (ROS) production, and elevation of intracellular calcium concentration. Similar neuroprotective action of Rbl in PC 12 cells treated with Aß(25-35) has been reported by a separate group of researchers [22], which is also related to the increased ratio of anti-apoptotic/apoptotic protein Bcl-2/Bax . Not only neuron but also astrocytes is protected by ginsenosides [23]. In cultured type I rat brain astrocytes (RBA), ginsenoside Rh2 inhibited Aß-induced inhibition of RBA growth, which is mediated by induction of pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP receptor PAC1 [24].
Anti-inflammatory effects
Due to the chronic neuroinflammatory responses occurring in AD and other neurodegenerative diseases, researchers investigated the anti-inflammatory effects of ginseng in brain. Several ginsenosides such as Rd, Rgl, Re, Rg3, Rlr2, and Rbl has been suggested to modulate neuroinflammatory responses in cultured microglia or stimulated brain [25-28]. In a mouse model of nemoinflammation induced by intracerebroventricular injection of Aß(l-42), ginsenoside Rbl reduced neuroinflammation as detected by cyclooxygenase-2 and inducible nitric oxide synthase (iNOS) expression. Remarkably, 4 wk treatment of Rbl at Are stage of eminent nemoinflammation by Aß(l-42) (2 wk after treatment), still effectively reduced inflammation along with the improvement of cognitive function as determined by Morris water maze test [29]. The anti-inflammatory effects of Rbl seem to involve NF-кВ pathway as evidenced by the modulation of Are level of IkB. More recently, strong anti-inflammatory effects of ginseng saponin metabolite, compound К [20-0-d-glucopyranosyl-20(S)-protopanaxadiol], have also been reported in two different in vivo models of neuroinflammation, i.e., sepsis and ischemia [30]. Not only the production of inflammatory mediators but also the recruitment of inflammatory cells into stimulated brain might be modulated by ginseng saponins, which might be regulated by Are modulation of expression of intercellular adhesion molecule-1 [31]. In rat model of inflammation, Rgl stimulated the production of NO and pro- inflammatory cytokines (interleukin [Ik]-Iß, IL-6, and tumor necrosis factor [TNF]-a), whereas Rbl exerted a significant inhibitory effect on the same proinflammatory mediators [32], which might suggest flrat Are regulation of inflammatory response by ginseng and ginsenosides is critically dependent on Are relative concentration and kinetics of treatment of individual components of ginseng.
The anti-inflammatory effects might be related to Are antioxidant property of ginseng. In a 7 mo chronic treatment experiment, it has been suggested Aral 100 or 200 mg/kg/d treatment of ginsenosides through drinking water unproved memory loss in senescence accelerated mice (SAMP8) along with increase antioxidant levels in serum [33]. The levels of plasticity related proteins including p-CAMKII, pCREB, and BDNF are increased in Are hippocampus of ginsenoside-treated mice aidrough it should be determined whether Are increased antioxidant activity has causal relationship with Are increased expression of plasticity related proteins as well as die improvement of memory in senescence accelerated mice.
Although much of the anti-inflammatory action of ginsenosides is dependent on the modulation of NF- кВ activity [26,29], anti-inflammatory activity of some of the ginsenosides such as R112 has been reported to be dependent on signaling pathways such as activator protein 1 and protein kinase A (PKA) [28,34] but not by NF-кВ [28]. In addition, regulation of inflammatory mediators such as cytokines and NO seems to be regulated by different signaling pathways, for example, mitogen- activated protein kinase and NF-кВ pathways in different cell lines [27,35]. These results suggest Aral Are regulation of cellular inflammatory response by ginseng and ginseng saponins is regulated by cell type and particular ginsenoside-specific manners, which necessitates fine dissection of die cellular signaling pathways involved.
Effects on overall Aß production
Using cell based assay system, which is a Chinese hamster ovary (CHO) cell line stably transfected with human APP 695wt, to detect the accumulation of Aß, Chen et al. demonstrated that certain ginsenosides lowered Aß concentration secreted into the culture supernatants in a dose-dependent manner [36]. The strongest effects were observed with ginsenoside Rg3 having an approximate IC50 of under 25 pM against Aß42. Among the various ginsenosides tested to be positive in the reduction of Aß, ginsenoside Rgl, Rg3, and RE resulted in significant reductions in die amount of Aß detected in die brains of an animal model of AD, which is Swedish mutant model of familial AD (Tg2576 line), after single oral administration, albeit small in extent compared with a known y-secretase inhibitor LY411575. Unfortunately, the authors can not provide what would be die actual molecular mechanism of the observed Aß-lowering effects of ginseng and ginsenosides.
As well as protecting cultured PC 12 cells from Aß- induced toxicity, a flavonol ganglioside isolated from roots of P notoginseng (RNFG) inhibited the aggregation of Aß in an in vitro assay in a dose-dependent manner [37]. It has been suggested that specific sugar moiety is required for the neuroprotective activity because the flavonol backbone was devoid of neuroprotective activity. No structural similarities to the currently available aggregation inhibitors have been suggested, which gives another level of complexity for tire understanding of tire possible protective mechanism of RNFG.
Effects on ß-secretase activity
The level of ß-site APP-cleaving enzyme 1 (BACE1) is responsible for tire elevation of Aß peptides in tire brain of AD patients and the expression of BACE1 can be regulated by peroxisome proliferator-activated receptor-y (PPARy) by binding to its promoter region. In N2a- APP695 cells, Rgl decreased tire levels of secreted Aß(l- 40) and Aß(l-42) as well as ß-CTFs, a cleaved C-tenni- nal fragment of APP by BACE1. The expression levels of both BACE1 mRNA and protein were decreased in cells treated with Rgl. Rgl induced activation of PPARy was demonstrated by tire nuclear translocation of PPARy and Rgl is suggested to have PPARy agonist activity like rosiglitazone [38]. In addition, Rbl inhibited BACE1 activity in vitro and rescue PC 12 cells from Aß(25-35)- induced toxicity as determined by LDH release, NO release, ROS production, lipid peroxidation, intracellular calcium elevation, and apoptosis [23]. Interestingly, Rgl treatment also inhibited activity of y-secretase in transgenic AD mice over-expressing APP/Aß (Tg mAPP) as well as В103-APP cells [39]. In addition, Rgl enhanced activation of PKA/CREB pathway suggesting the multitarget action of Rgl against AD. In a more recent study using human platelets, it has been demonstrated that Rgl promoted a-secretase cleavage of APP via estrogen receptor (ER) extranuclear signaling pathway suggesting Rgl behaves as phytoestrogen, which might have important implications considering estrogen withdrawal as a risk factor for AD [40,41].
Aß clearance
Although most of tire studies dealing with tire role of ginseng on glial cells have focused on tire anti-inflammatory activity, Joo and Lee [42] focused on tire phagocytic role of microglia and found that Rg3, a by-product of red ginseng, enhances tire microglial Aß uptake, internalization, and digestion. The increased clearance of Aß by microglia by Rg3 seems to be mediated by tire increased expression of macrophage scavenger receptor type A, which may provide an additional therapeutic target of AD by ginseng.
In SK-N-SH cells transfected with SweAPP, ginsen- oside Rg3 reduced tire level of AMO and Aß42, probably due to the increase in gene expression of neprilysin [43], which has been suggested to be tire rate-limiting enzyme in the Aß degradation in tire brain [44]. Whether other proteases such as martix metalloproteinase, angiotensin converting enzyme, and tissue plasminogen activator, which has been implicated in Aß clearance, were also affected by ginsenoside remains to be investigated. At present, the data investigating whether ginseng and gin- senosides may affects Aß-clearance through transport across tire blood-brain barrier by modulating tire regulator molecules such as receptor for advanced glycation end products and lipoprotein receptor-related protein are not available, which may need further investigation.
Effects on tau phosphorylation
Increased phosphorylation of tau protein can results in self aggregation, which is involved in tire pathogenesis of AD. After exposure to Aß(25-35), the levels of tau protein phosphorylation in hippocampal neurons at the sites of Thr205, Ser396, and Ser404 were increased as well as the level of p25. Pretreatment with ginsenoside Rbl, at least in part, reversed these changes [45]. Similarly, Rbl reduced tire phosphorylation of tau induced by Aß(l-42), which is regulated by a cascade of signaling pathways comprised with PI3K-Akt-GSK3ß [46]. Total ginsenosides extracts also partially inhibited cyclosporine-induced increase in tau phosphorylation in SY-5Y cells [20]. Using tire slice culture of brain obtained from 5 weeks old Wistar rats, it has also been suggested that ginsenoside Rglsignificantly inhibited okadaic acid- induced phophorylation of tau [47]. The same group of researchers also reported that pretreatment of ginsenoside Rd inhibited okadaic acid-induced tau phosphorylation in cultured neuron as well as in brain of male Sprague Dawley rats bilaterally micro-infused with okadaic acid into tire cerebral ventricle, which might be mediated by tire activation of PP-2A [48].
Effects on cholinergic system
Loss of cholinergic neurons in cerebral cortex and hippocampus is closely associated with AD. Panaxynol, one of the compounds isolated from tire lipophilic fraction of P nologinseng. concentration-dependently up-regulated tire number of M, receptor in CHO cells transfected with human m, subtype gene [49]. Panaxynol caused a significant stimulation of cAMP accumulation and tire increase in M| receptor number was blocked by a PKA inhibitor RP-cAMP. In PC 12 cells it has been suggested that Rbl and Rgl increased neurotransmitter release by modulating the phosphorylation of synapsin in PKA dependent and independent manner, respectively [50].
Ginsenosides also modulate acetylcholine release and the level of choline acetyl transferase (ClrAT). Rbl and Rgl can modulate acetylcholine release and reuptake determined with rat hippocampal slices [51] and the number of choline uptake sites determined by [3H] hemicholinium-3 binding experiments, especially in tire hippocampus and to a lesser extent in cortex when it administered at least three days [52]. They also increased choline acetyltransferase levels in rodent brains which was determined by in situ hybridization in basal forebrain [53] as well as the level of acetylcholine in the brain [54], although other reports suggested Rgl did not show any effects on ClrAT activity [51]. Interestingly, Aß(25-35)- induced suppression of the К-evoked [3H]-acetylcholine release from the rat hippocampal slices was effectively reversed by Rbl but not by Rgl in cholinergic synapse [55]. Taken together, these data suggest that Rbl is the principal components regulating acetylcholine dynamics in brain.
Human clinical trials
In healthy volunteers, acute or chronic treatment of ginseng extracts produced both positive and negative results in cognitive functional tests (for a review, see [6,56]), preventing a generalized conclusion on the psychoactive properties of ginseng. Nevertheless, a few clinical trials have been conducted regarding the efficacy of ginseng or ginsenosides on AD. In 12 wk, open-label clinical trial, Heo et al. [57] treated AD patients with low or high concentration of Korean red ginseng extracts for 12 wk. The congnitive function of patients was assessed using the Alzheimer's Disease Assessment Scale, Korean version of the Mini-Mental Status Examination and Clinical Dementia Rating scale at the end of the 12 wk study period and high concentration of Korean red ginseng extracts treated group showed either a tendency or statistically significant improvement as compared with control group. The same group of researchers elaborated further with more patients and reported that 12 wk treatment of encapsulated P. ginseng powder (4.5 g/d) increased cognitive performance and tire discontinuation of ginseng gradually decreased tire congnitive performance back to control level [58]. Although the number of subjects is relatively small, tire results suggest tire potential of therapeutic significance of ginseng compounds in AD.
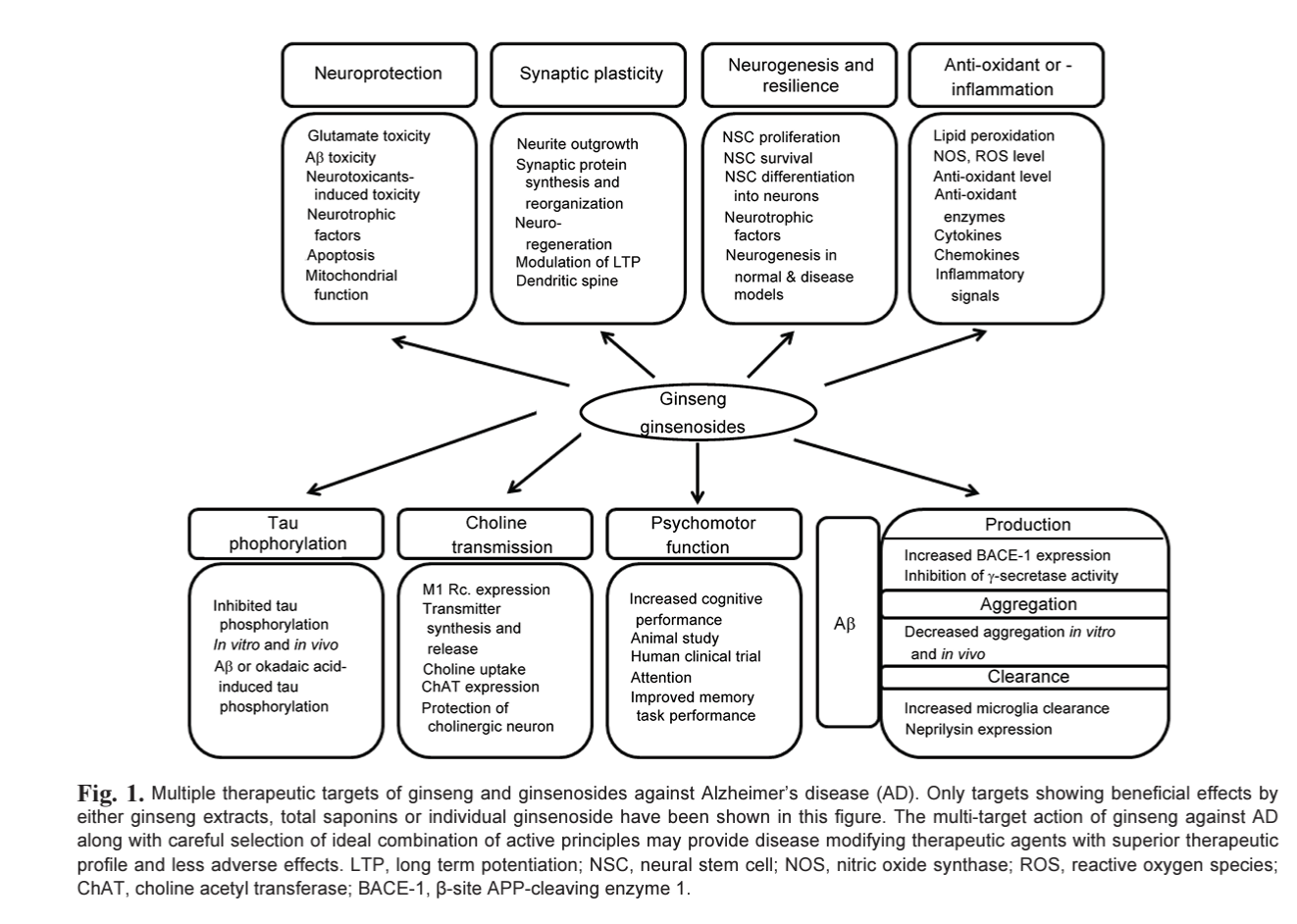
Currently available drugs and many of the experimental reagents against AD are generally symptomatic treatment and disease modifying medication is eagerly anticipated. In general, aggregation blockers and reagents effective for the Aß dynamics in brain as well as drugs targeting Aß production and clearance are expected to possess disease modifying potentials in some extents. Some of ginseng components are suitable for this criteria but the investigation in this field still in its infancy albeit numerous reports suggesting the beneficial effects on cognition and neuroprotection. In addition, some ginsenosides seemingly have multiple site of action, which make them ideal candidates for tire next generation AD therapeutics. Future will tell, with unceasing efforts from existing researchers as well as new investigators in this field, whether the multi-faceted effects of ginsenosides on AD will be proven successful in the long run (Fig. 1).
PARKINSON'S DISEASE
Mainly due to the strong antioxidant effects and neu- roprotective effects of ginseng and related compounds, many researchers investigated the effect of ginseng on neuroprotection in culture or annual models of PD. Some of the early studies using cultured neuronal cell lines such as PC 12 suggested ginseng and ginsenosides provided anti-oxidant effects thereby protecting the cells from oxidant injury induced by dopamine [59], hydrogen peroxide [60] or l-methyl-4-phenyl-l,2,3,6-tetrahydropyridine (MPTP). Treatment of dopamine in a concentration of 0.15 to 0.45 mM for 24 h induced apoptotic cell death of PC 12 cells and tire pretreatment of ginsenoside Rgl protected apoptotic cell death. Ginsenoside Rgl reduced tire generation of dopamine-induced reactive oxygen species, iNOS induction and NO release, and the release of mitochondrial cytochrome c into tire cytosol, and subsequently inhibited tire activation of caspase-3 [59].
Some researchers also used primary dopaminergic cultures obtained from embryonic mouse mesencephalon and showed partial neuroprotective effects of ginsenoside Rgl and Rbl as evidenced by inhibition of decreased neurite length or number induced by a toxic metabolite of MPTP, l-methyl-4-phenylpyridinium (MPP) treatment [61]. Somewhat bigger neuroprotective effects of ginsenoside Rgl has also been demonstrated in reducing MPTP-induced substantia nigra neuronal loss in С57/ BL6 mice, possibly via increased level of glutathione and superoxide dismutase activity [62]. Rgl also inhibited MPTP-induced overload of iron and modulation of the expression of iron transporters such as divalent metal transporter and ferroportinl in tire substantia nigra [63]. The activity and level of iron transporters is regulated by the IRE dependent transcriptional control as well as inhibition of iron regulator protein [64], which seems to be mediated by the regulation of ROS-NF-кВ pathway [65]. Consistent with these results, it has been reported that panaxatriol saponins extracted from P. notoginseng induced thioredoxin-1 and protect PC 12 cells or Kunming mice from MPP+ or MPTP-induced toxicity, which was determined by MTT assay, LDH release and behavioral assays such as locomotor activity test and fraction test [66,67]. Similar neuroprotective effects was also reported using rotenone model of neurotoxicity using primary dopaminergic neuron [68], which involves inhibition of mitochondrial apoptotic pathway, possibly via the modulation of glucocorticoid receptor and PI3K/Akt pathway. Involvement of Akt pathway in the modulation of neuronal survival by Rgl against 6-hydroxydopamine (6-OHDA)-induced cell death was also reported in a study using MES23.5 cells [69]. Rgl modulated 6-OH- DA-induced loss of mitochondrial membrane potential and apoptotic protein expression in human neuroblastoma SK-N-SH cells via modulation of estrogen receptor as evidenced by increased estrogen responsive element (ERE)-luciferase activity by Rgl treatment in cells transfected with the ERE-luciferase reporter construct [70], suggesting the phytoestrogen-like effect of Rgl. Pharmacological study also suggested the involvement and possible crosstalk between ER and insulin-like growth factor receptor pathways in Rgl-mediated nemoprotection in 6-OHDA treated SK-N-SH cells [70]. Similar findings were confirmed in vivo using 6-OHDA lesion model by unilaterally injecting 6-OHDA into the medial forebrain bundle of ovariectomized Wistar rats [71].
In spite of seemingly strong evidence that ginsenoside Rgl shows strong antioxidant effects in these PD models, a recent study suggested that individual ginsenosides such as Rbl, Rgl, and Re, have stimulus-dependent effects on tire regulation of CD40 expression and virtually have no effects on NO production in N9 microglia, while P. notoginseng extracts showed strong inhibitory effects on CD40 expression, cytokine expression, NO production, and chemokine receptor expression regardless of the identity of immunological stimulus either by TRL ligands such as LPS and poly I:C, or by cytokine such as IFN-y, suggesting it may be tire combination of different ginsenosides but not a single ginsenosides that provide strong anti-inflammatory efleets against TRL ligand or cytokine stimulation of microglia [72]. In this study, it is also suggested that tire anti-inflammatory effects of P notoginseng extracts is not mediated by glucocorticoid receptor given that a pharmacological inhibitor of glucocorticoid receptor, RU-486, failed to reverse the antiinflammatory effects of P notoginseng extracts [72].
Animals received oral administration of P ginseng extract Gil5 10 d prior to and/or following exposure (total 20 d) to Are MPTP in mice, or MPP+ in rats showed significantly fewer tyrosine hydroxylase (TH)-positive cell loss along with the reduction of apomorphine-induced contralateral rotations [73]. The lowest effective dose was 75 mg/kg/d and 200 or 500 mg/kg/d almost completely abolished tire dopaminergic neural cell death in substantia nigra. Interestingly, tire administration of Gil 5 can be delayed until 2 d after MPP+ administration, which still showed modest protective effects [73]. The delayed protection conferred by Gil5 is not likely due to blockade of neurotoxin uptake. Rather, the authors suggested the neurotrophic effects of ginseng such as tire induction of NGF [53,74] may underlie tire observed neuroprotective effects.
Although the majority of PD etiology is believed to be sporadic, genetic defects also contribute in the pathogenesis of PD and tire annual models based on the genetic modulation of key genes involved in PD pathogenesis such as LRRK2 and a-synuclein found in autosomal- dominant PD as well as parkin, DJ-1, and PINK1 responsible for autosomal-recessive PD, provided insight into tire molecular mechanisms of PD and new opportunities to design and develop new therapeutic agents for this devastating disease [75]. The investigation of the therapeutic effects and molecular and cellular mechanisms of ginseng on these animal models will provide further evidence of the efficacy of ginseng in PD.
OTHER NEURODEGENERATIVE DISORDERS
Huntington's disease
HD is an autosomal dominant, inherited disorder characterized by progressive nemodegeneration resulting in motor abnormalities, including chorea and psychiatric disturbance with gradual dementia. The characteristic pathological feature of HD is a polyglutamine expansion in huntingtin and tire loss of medium spiny neurons in the striatum, although tire exact mechanism of pathophysiology needs to be clarified. HD is an incurable disease, which needs the development of effective therapeutic reagents. Using in vitro culture of medium spiny neuron of striatum obtained from fire YAC128HD mouse model, Wu et al. [76] tested ginsenosides such as Rbl, Re, Rd, Re, Rg3, Rg5, Rhl as well as Re and Rd mixture, Rkl and Rg5 mixture, and Rh4 and Rk3 mixture. The authors reported that nanomolar concentrations of ginsenoside Rbl and Rc and micromolar concentration of Rg5 effectively protected TAG 128 medium spiny neurons from glutamate-induced toxicity, which was mediated by regulation of intracellular calcium concentration. Other ginsenosides were without effects. Unfortunately, follow up studies examining the effects of those ginsenosides on other models of HD as well as the effects in animal models are not available yet, which will substantiate fire efforts to develop HD medication based on ginseng and related products.
Amyotrophic lateral sclerosis
ALS is a degenerating motor neuron disease characterized by muscle weakness and paralysis in affected individuals. The correct pathophysiology is currently unknown with which most of fire cases are sporadic with a few identified genetic risk factors. Albeit these lack of pathophiological information of the disease, it has been reported that mutations in fire gene that produces tire Си/ Zn superoxide dismutase (SOD1) enzyme were highly associated with familial cases of ALS and experimental animals with SOD1 mutation showed ALS-like phenotypes. Using B6SJL-TgN(SODl-G93A)lGm transgenic mice, the efficacy of ginseng extracts, which was given in drinking water, was tested on onset of motor symptoms as well as survival rates of tire mice [77]. Although there was no dose-response relationship (40 and 80 mg/ kg), ginseng extracts provided weak but significant delay in Are onset of ALS symptoms when it administered from age 30 d and onwards [77]. Whether the protective effects are related to the anti-oxidant effects (and thereby nemoprotection) or neurotrophic effects of ginseng remains to be determined.
CEREBRAL ISCHEMIA
Protective effects of ginseng and ginsenosides
There are dozens of studies indicating ginseng extracts or ginsenosides obtained from various ginseng species provide functional as well as neuroanatomical protection from ischemic stroke (Table 1). Various experimental models has been used including middle cerebral artery occlusion (focal transient ischemia), four-vessel or bilateral common carotid artery occlusion (global ischemia)
Table 1. Effects of ginseng or ginsenosides on experimental ischemic stroke
Experimental scheme | Species | Treatment protocol | End point | Reference |
Focal ischemia |
| |||
MCAO (30 min) | Rat (Swiss albino adult male, 200-250 g) | Pre-treated with Korean ginseng tea (350 mg/kg given orally for 10 d) | Histopathology Brain weight and water contents Oxidative stress | [78] |
MCAO MCAO | SD (270-320 g) | Rd (50 mg/kg), i.p. 30 min pretreatment Rd (10-50 mg/kg), same as above | Infarct volume, mitochondrial function, apoptosis Inflammatory responses, oxidative stress | [89] [90] |
MCAO (60 min) | C57BL/6 (16-18 mo) | Rd (10-50 mg/kg), same as above | Oxidative stress | [85] |
MCAO (2 h) | SD (250-300 g) | Rd (10 mg/kg) i.p. injected 15 min before MCAO | Expression of cation channels TRPM7 and ASIC la | [91] |
MCAO (2 h) | Rat | PNS (50 mg/kg), i.p. 2 h after reperfusion | Expression of intercellular adhesion molecule-1 Brain neutrophil infiltration Brain edema, neurological score, infarct size | [31] [92] |
MCAO (2 h) | SD (300 g) | KRG (100 mg/kg/d) after reperfusion. Once daily for 1 wk. Oral treatment | Infarct volume, neurological score, serum cytokine level | M |
MCAO (2 h) | SD (270-320 g) | PNS (25 mg/kg), i.p. 5 min before and 12 h, 24 h and 36 h after MCAO | Apoptosis (TUNEL staining, caspase 1, 3 expresison) | [94] |
MCAO (2 h) | SD (250 g) | Intranasal Rbl (1.25 mg/kg or 12.5 mg/kg) | Infarct volume, neuronal density Autophagic marker protein level (LC3, Beclin 1) after 24 h reperfusion | [95] |
MCAO (2 h) | SD (220-250 g) | Rbl (12.5 mg/kg/d) intranasal once daily, 7 d before MCAO | Neuroinflammation (microglial activity, increased production of TNF-a, IL-6, and activation of NF-кВ) | [96] |
MCAO (2 h) | SD (270-280 g) | Rh2, 100 mg/kg, oral treatment immediately prior to reperfusion | Infarct volume, neurological score after 22 h reperfusion | M |
MCAO (2 h) | SD (250-280 g) | Black ginseng extracts (100 or 400 mg/kg), oral (daily for 2 wk) after ischemia | Neuronal density Learning and memory (Morris water maze) Cholinergic system (ChAT), nNOS expression in hippocampus | [97] |
MCAO (30 min) C57BL/6 mice (10-11 weeks olcT | Compound К (30 mg/kg i.p.) 4 d before ischemia I | Infarct volume, microglial activation | [30] | |
MCAO | SD (270-305 g) | PGE (200 mg/kg), oral treatment for 1 wk after ischemia | Infarct volume, behaviors after 15 d (rota-rod and adhesive removal test), activating astrocyte, neuronal death, and ap opto tic cell death | [98] |
MCAO | SHR-SP | Dihydroginsenoside Rbl (dgRbl) i.v. after ischemia and | Infarct volume (24 h and 4 wk) | [99] |
(permanent) | (250-300 g) | then infused using osmotic pump (0.6 or 6 g/d) Rbl, i.v. immediately or 2 h after ischemia, then chronic infusion, as above (6, 60, 3000 or 12,000 g/d) Rbl, i.v. 2 h before or immediately after MCAO and then chronic infusion as above | Learning and memory (Morris water maze) Infarct volume, learning and memory (Morris water maze) and neuronal staining | [87] [100] |
MCAO (90 min) | SD (280-320 g) | PNE (50 mg/kg) i.p. 2 h after the onset of MCAO | Infarct volume and microglial activation 24 h after reperfusion | [101] |
MCAO (1 h) | SD (250-300 g) | Rg2 (2.5, 5 and 10 mg/kg), 15 min or 24 h after ischemia, Neuronal density, infarct volume, Neurological score, Y-maze i.v. into tail vein test, Expression of apoptosis-related proteins | [102] | |
MCAO (2 h) | Rat | Rbl (10-40 mg/kg), i.v. 30 min before or immediately after MCAO | infarct size, neurologic deficit, contents of calcium and potassium in the infarct | [103] |
MCAO (permanent) | Rat | Rbl (40 mg/kg), i.v. | infarct size, neurologic deficit, contents of calcium and potassium in the infarct | [103] |
MCAO (permanent) | Wistar | PGS (25mg/kg/d), i.p. 3 day before ischemia until 1-14 d | Neurological score Neurogenesis (BrdU and NeuN staining) | [104] |
Global ischemia |
| |||
4-VO (10 min) | Wistar, male, 160-180 g | PGE (Ethanolic, 200 mg/kg), i.p. 0 and 10 min after ischemia | Hippocampal neuronal protection (Nissl staining, lipid peroxi da- | [79] |
BCCaO (30 min) | Rat (Swiss albino pre-treated with Korean ginseng tea (350 mg/kg given adult male, 350 g) orally for 10 d) | Histopathology, Brain weight and water contents Oxidative stress | [78] | |
BCCaO (5 min) | Gerbil (male, 70-80 g) | Pretreatment (7 d) red ginseng powder (oral, 1.5g/kg), total saponin (i.p. 100 mg/kg), Rbl (i.p. 20 mg/kg). Rgl and Ro were ineffective | Hippocampal CAI protection. Electron microscopy Memory improvement (passive avoidance) | [81] |
BCCaO (3- 3.5 min) | Gerbil (male, 70-80 g) | Rbl (2.5 or 25 ng), i.c.v. injection immediately after ischemia | Neurological deficit (passive avoidance test) Neuronal density in hippocampal CAI field | [105] |
BCCaO (3 min) | Gerbil (male, 70-80 g) | Rbl (2.5 or 25 ng), i.c.v. injection into the left lateral ventricle and then Rbl (60 or 600 ng/day) was continuously infused for 7 days into ventricles | TUNEL staining Bcl-xL expression | [87] |
4-VO (40 min) | Rat | Ginsenosides Rb + R0 100 mg/kg i.v 30 min before ischemia | prostacyclin synthesis, thromboxane A2formation and lipid peroxidation, creatine phosphokinase, brain edema (1 h reperfusion) | [80] |
4-VO | Rat | Ginseng (200 mg/kg/d) 1 wk before the occlusion | Recovery of local cerebral glucose utilization | [106] |
BCCaO + right MCAO (90 min) | SD | PNE (water), oral, 0.5 g/kg/d, 3 d a week for 4 wk after the operation | Neurological score, learning and memory (eight-arm radial maze), BDNF, //-seceretase and immunological markers | [107] |
In vitro |
|
|
|
|
OGD (2 h) | Hippocampal neuron (El 8, SD) | Rd (0.1-10 цМ) during and after OGD | Cell death, oxidative stress, mitochondrial function | [84] |
OGD (10 min) | Acute hippocampal slice (adult Kunming mice) | Rb3 (10-50 pM) 20 min prior to and during OGD | The recovery of the amplitude of population spike (PS) in the stratum pyramidale | [108] |
Hypoxia (0.1% O2) | Human neuroblastoma cells SK-N-MC | PGE, 100 mg/mL, 6 h | Hypoxia-induced cell death Gene expression profile (human 8К microarray) | [109] |
OGD (6 h) | Primary hippocampal neural stem cell (SD, 1 day old) | PNS (4.4 pg/mL-2.2 mg/mL) | Proliferation (BrdU) Differentiation into neuron and glia | [110] |
OGD (4 h) | PCI 2 | Rb3 (0.1-10 M) | Cell death, apoptosis, calcium elevation, caspases and apoptosis related protein expression | [111] |
The effects of Panax ginseng or P notoginseng either in extracts or total saponin forms as well as ginsenosides obtained from them on animal or culture model of ischemia were summarized. Experimental scheme was categorized as focal and global ischemia as well as in vitro culture model. The treatment protocols of ginseng and experimental end points in each experiment were also summarized with relevant references.
MCAO, middle cerebral artery occlusion; SD, Sprague-Dawley rats; PNS, P notoginseng saponins; KRG, Korean red ginseng; TNF, tumor necrosis factor; IL, interleukin; Ch AT, choline acetyl transferase; nNOS; neuronal nitric oxide synthase; PGE, P ginseng extracts; SHR-SP, spontaneously hypertensive rats-stroke prone; PNE, P notoginseng extracts; PGS, P ginseng saponins; 4-VO, four-vessel occlusion; BCCaO, bilateral (common) carotid artery occlusion; OGD, oxygen glucose deprivation in rats and mice [78-80], transient forebrain ischemia by short-term transient bilateral common carotid artery ligation in gerbil [81], and hypoxic-ischemic brain injury model in neonatal rats [82], in addition to tire in vitro oxygen glucose deprivation or glutamate-induced toxicity model [83,84]. For example, ginsenosides Rd [85], Rg3 [86], Rb and Ro [80,87] as well as ginsenoside Rh2 reduced ischemic brain injury in rats or mice [88]. In 5-min transient forebrain ischemia model of gerbil, ginsenoside Rbl significantly prolonged the response latency of ischemic gerbils in passive avoidance test and rescued a significant number of ischemic CAI pyramidal neurons, whereas ginisenosides Rgl and Ro were ineffective.
Anti-ischemic mechanism of ginseng and ginsenosides
Although part of the reason that much of the pharmacological intervention study against neurological diseases using ginseng and/or ginsenosides are focused on cerebral ischemia are related to tire ginseng's potent antiinflammatory and anti-oxidative activity in microglia and astrocytes as well as in insulted brain [30,80,101,112], many other studies suggest that other mechanisms are also involved in the potent neuroprotective or neurorestorative actions such as regulation of channel proteins including TRM, ASIC and NMDA receptors [91], Potassium channel [113], Na channel [114], inhibition of mitochondrial permeability transition pores [86], regulation of anti-apoptotic proteins [87], changes in receptor binding activity including NMDA and GABA receptors [115], defense against excessive endoplasmic reticulum stress [116], regulation of angiogenesis and the expression of vascular endothelial growth factor [99,117,118], cerebral vasorelaxation possibly via NO pathway [119], and induction of hypoxia inducible factor-la [120], which may affects cell survival, angiogenesis, and nemogenesis after ischemic injury.
In one study, rats were subjected to 45 min of myocardial ischemia followed by 120 min of reperfusion and 10 min preconditioning with ginsenoside Rbl right before tire induction of ischemia increased Akt phosphorylation, probably through the activation of the PI3K pathway [121]. РІЗК/Akt pathway has been shown to be essential in the regulation of cell survival as well as infarct size
reduction, which seems to be plausible molecular switch mediating the effects of ginseng in ischemic stroke in brain as well.
Excessive sodium influx is one of the factors involved in neuronal damage in ischemic conditions. In tsA201 cells transfected with cDNA expressing a subunits of tire Brain2a Na+ channel, the whole-cell patch clamp experiments revealed that American ginseng (P. quinquefolius) extract or ginsenoside Rbl, tonically and reversibly blocked the channel in a concentration- and voltagedependent manner [114]. Ginsenoside Rbl stimulated tire expression of tire mitochondrion-associated antiapoptotic factor Bcl-x(L) in vitro as well as in stroke prone spontaneously hypertensive rats (SP-SHR) subjected to permanent focal cerebral ischemia [87]. Interestingly, Stat5 responsive element in tire bcl-x promoter became active in response to Rbl treatment suggesting tire possible role of Rbl in tire regulation of cell death pathway thereby providing nemoprotection in ischemic condition [87].
Ginsenosides can regulate the expression of factors important in tire pathogenesis and prognosis of ischemia such as HIF-la. The induction of HIF-la by ginsenosides is independent of hypoxia, for example, ginsenoside Rgl regulated the induction of HIF-la even in nonnoxic condition [120]. Interestingly, tire induction is not regulated by transcriptional regulation evidenced by no changes in the level of HIF-la mRNA. Rather, the induction is governed by translational up-regulation of HIF-la in a mechanism dependent on tire activation of PI3K and S6K pathway. LY294002 or rapamycin, inhibitors of PI3K and S6K pathway, respectively, attenuated Rgl-mediated induction of HIF-la, suggesting tire essential role of tlrese pathways in the ginsenoside-mediated regulation of HIF-la. Considering the importance of mTOR pathw ay in several psychiatric disorders, this data is potentially interesting to investigate w hether regulation of mTOR is involved in flic regulation of psychiatric disorders by ginseng.
Ginseng and ginsenosides may affect nemogenesis in flic ischemic brain. It has been suggested that ginsenoside Rgl and Rbl increase proliferation and differentiation of neural progenitor cells in dentate gyrus of hippocampus of normal adult mice and gerbils subjected to global ischemia suggesting the usefulness of the ginsenosides in neurodegenerative diseases such as AD as well as neurological insults condition such as cerebral ischemia (for a review, see [16]). P notoginseng extracts increased neural stem cell (NSC) proliferation and tire expression of nestin/BrdU, and also enhanced Tuj-1, vimentin, and nestin mRNA expressions in hippocampal NSCs isolated from newborn rat hippocampus with similar effects in oxygen glucose deprived NSCs, suggesting possible neu- roprotective role of ginseng by providing increased neurogenesis in ischemic or neurodegenerative conditions [110]. Similarly, when total ginseng total saponin (GTS) was intraperitoneally administered from 3 d before ischemia until 14 d after it, the number of BrdU+ cells and BrdU+/NeuN+ cells in GTS group were significantly higher than those in saline-treated group in tire ipsilateral subventricular zone and in tire ipsilateral infarct area after MC AO [104]. Importantly, the increase of tire number of BrdU+/NeuN+ cells highly correlated with tire decrease of neurological scores, suggesting the therapeutic role of tire increased nemogenesis by GTS in ischemic stroke.
More recently, it has been suggested that tire nemopro- tective effects of Rb3 in oxygen-glucose deprived neuron might be related to their effects on GABAa receptor [108]. It remains to be determined whether the GABAergic effects of Rb3 are dependent on the modulation of steroid binding sites on GABAa receptor.
Unfortunately, most of the studies in this field me explanatory on the therapeutic potential of ginseng compounds with little explanation of mechanism of action, especially, the causal relationship of the molecular or biological changes induced by ginseng on therapeutic action.
Recently, Yue et al. [122] reported that eighteen proteins involved in pathways including energy metabolism, lipid metabolism, muscle contraction, heat shock stress, and cell survival and proliferation has been changed in ischemia-reperfusion induced injury model of rat treated with notoginsengnosides with or without salvianolic acids suggesting these proteins might be the possible protein targets in their cardioprotective effects. Some of these proteins me also implicated in nemoprotection as well as ischemia-induced neuronal cell death pathway, which may prompts further study using cerebral ischemia model.
Although it has been reported that ginseng regulates a set of gene which is involved in cellular physiologic response related genes such as MPHOSPH10, IMP-3 and SDCBP, in hypoxic human nemoblastoma cells SK-N- MC in an experiment using 8K human cDNA microarray analysis [109], systemic investigation trying to find out cellular responses affected by ginseng using metabolo- mics, proteomics, and genomics approach in ischemic condition is scarce. In this sense, it is noteworthy that two studies investigated the effects of ginseng in gene expression profile after immobilization stress (Gene Expression Omnibus [GEO] accession number GSE12656) and tire role of micro RNA in ginsenoside-Rgl-induced Angiogenesis (GSE17541) [117,118], with their data submitted in GEO.
Clinical trials and human study
There's not much carefully designed, controlled clinical trials using ginseng on the possible effects on ischemic stroke. Sanchi, the dried root of P notoginseng, is one of the most widely used herbal medicines for ischemic stroke in China and Chen et al. [123] examined available clinical reports about the effectiveness and safety of Sanchi and concluded that Sanchi appears to be beneficial and safe for acute ischemic stroke. However, as the authors stated in their review, the small sample size and inferior quality of study design and performance in some of those studies, prevented a definite conclusion, which dictates more well-designed randomized controlled trials. Recently, one clinical trial has suggested that со-treatment of Sanchi capsule with low concentration of aspirine significantly ameliorated neurological deficit and impairments in activities of daily living in a study conducted with 140 human patients hospitalized in four hospitals in China over the year 2004 to 2006 [124].
Kinetics and related issues
In most of the studies, ginseng extracts or ginsenosides were treated at least 30 min before the induction of ischemia [80,81,96,106]. In many cases, post ischemic treatment of ginseng or ginsenoside was not effective to rescue the ischemic brain [81], which limits the therapeutic applicability of the ginseng and related products. However, the post-ischemic infusion of ginsenoside Rgl provided neuroprotection as well as functional recovery determined by Morris water maze after permanent middle cerebral artery occlusion in SP-SHR [87]. In some cases, ginseng containing prescription such as Sheng- mai San also effectively suppressed the oxidative stress determined by TEARS formation even when it was administered after 45 min reperfusion following ischemia, although individual components of tire prescription did not provided a protective effects [125]. Recently, it was also reported that treatment of Korean red ginseng (KRG) after ischemic injury protected brain from neurological deficits [93]. In this case, the authors used adult male Sprague-Dawley rats to induce transient middle cerebral artery occlusion for two hours and then the rats were fed KRG extract (100 mg/kg/d per orally) or saline after reperfusion. After seven day treatment of KRG extracts, both brain infarct volume as well as neurological score was improved by KRG treatment and the elevated serum levels of TNF-a, IE-IP, and IL-6 were attenuated by KRG with concomitant increase in serum level of IL-10 [93], which has been argued to be responsible for the improved ischemic outcome with oral-treatment of KRG extract. In one study using transient focal cerebral ischemia (MCAO) model in rats, P notoginseng saponin (PNS) was administered at different time points after MCAO and the authors argued that the administration of PNS at 3 to 4 h after onset of ischemia significantly reduced neurological deficit score, infarct size and brain edema with decreasing effects at 5 h after tire onset of ischemia and no effects at 6 h suggesting the therapeutic window until 5 h in rat model [92].
Intravenous injection of Rbl before or with MCAO at dose range of 10 to 40 mg/kg in rat reduced infarct size and neurological deficit score [103] but not with 40 mg/ kg PNS [126], suggesting the use of correct combination or active principle of ginsenosides might provide lower therapeutic dose for tire treatment of ischemia.
Another point of concern in tire comparison of different reports regarding tire effectiveness of ginseng is the route of administration. Many of studies used IV injection of ginsenosides for tire study of short term effects of ginsenosides [80], while others used oral administration route to study the long term effects or the restorative potential of ginseng. Even in some cases, ginsenoside was administered by intracerebroventricular infusion [105]. Intranasal delivery of ginsenoside Rbl targets the brain and ameliorates cerebral ischemia/reperfusion injury in rats [95], which might be a good route of administration to overcome tire blocking effects of blood brain barrier against ginsenoside. In this study, it was argued that a local bioavailability of 10.28% to 32.48% and drug targeting index (which means tire availability of Rbl in brain compared with IV injection) of 7.35 to 23.22 in different brain regions. Intranasal Rbl was determined to be braintargeting and might be an efficient way to deliver ginsenosides in cerebral ischemia.
DEPRESSION
Several reports suggested that traditional medicinal formulation containing ginseng may be effective in ameliorating tire symptoms of depression in humans and animal models [127-129]. In addition, total ginseng extracts or total saponin preparation has been reported to be effective in combating depression-like behaviors evaluated using annual models such as tail suspension test, forced swimming test, injection of corticosterone, chronic unpredictable mild stress, and menopausal depressive-
like state in female mice induced by ovariectomy or morphine withdrawal-induced depression behavior [130- 136]. Similar anti-depressive effects of chronic oral KRG extracts administration on human postmenopausal patients with climacteric syndrome have also been reported [137].
Based on the anti-depressant effects of orally administered ginseng, Xu et al. [138] examined the effects of intestinal metabolite of ginseng, 20(S)-protopanaxadiol (code name Sill) on depressive behavior in experimental annuals, which was tested in tail suspension test and forced swimming test as well as olfactory bulbectomy depression model in rats. Interestingly, the antidepres- sant-like activity was comparable to that of fluoxetine and Sill increased monoamine neurotransmitters in the brain and showed modest inhibitory effects against neurotransmitter reuptake in vitro. In contrast to fluoxetine, Sill inhibited oxidative stress and reduced serum corticosterone level in olfactory bulbectomized animals suggesting Sill modulates depression-like behavior by regulating multiple targets including both central and peripheral inflammation, regulation of hypothalamic corticotrophin-releasing factor (CRF) and Neuropeptide Y (NPY) expression and regulation of glucocorticoid receptor and BDNF expression as well as neurofilament- L in hippocampus [133-135,139]. Many of these results suggest that ginseng and ginsenosides regulate brain neurotransmitter turnover and hypothalamic- pituitaryadrenal axis.
Ginsenosides may affect adult nemogenesis which is reminiscent of the effects of fluoxetine on hippocampal nemogenesis implicating a possible link of the ginseng and related compounds on anti-depressant action. Actually, in a recent experiment using chronic mild stress model of depression in mice, it has been reported that Rgl up-regulated the BDNF signaling pathway in the hippocampus and reversed the decrease in dendritic spine density and hippocampal nemogenesis caused by chronic mild stress without affecting Are monoaminergic system [15]. Recently, Yamada et al. [136] reported that Rg3 and compound К provided strong anti-depressant effects in ovariectomized animals which me blocked by 5-HT2A antagonist ritanserine suggesting additional moleculm target in Are treatment of depression by ginseng.
ANXIETY
In several different experimental paradigms to detect anxiety like behaviors such as open-field, elevated plus-maze tests, conflict behavior in thirsty rats and footshock-induced fighting in paired mice, chronic oral administration of ginseng extracts but not single acute administration of ginseng produced comparable anxiolytic activity as acute diazepam [140], which is suggested to be related with the modulation of monoamine oxidase activity. Using five day-old male chick, the effects of ginsenoside Rbl on separation distress was evaluated, which provided robust effects of ginseng on anxiety level. These results led Are authors to draw the conclusion Arat nootropic hence Are memory enhancing effects of ginseng is strongly associated with the anxiolytic effects. Among several different ginsenosides tested including Rbl, Rgl, Rg3-R, and Rg3-S, and Are Rg5 and Rk, ginsenosides Rbl, Rgl, and Are Rg5 and Rk mixture showed anxiolytic-like effects in elevated plus maze test. Similar anxiolytic effects of Rbl [143] or Rg3 and Rlr2 was observed in separate sets of experiments [144]. Although the anxiolytic activity of ginsenosides may involve the action on GABA/Benzodiazepine receptors [144], ginsenosides inhibited locomotor activity to a lesser extent Aran diazepam, which may implicate Are better side/adverse effect profile of ginsenosides as anxiolytic agents [142,145].
However, negative results me also available that did not observe any effects on anxiety or depression behavior even after 3 wk chronic treatment of several ginsenosides [146], which suggests that careful interpretation and more studies might be needed using multiple annual models and test paradigms including light/dmk test, holeboard test, and isolation-induced aggressive test [145], which is reviewed in a recent article [147].
ADDICTION
Ginseng has been believed to reduce the behavioral and physiological responses against psychostimulants and other drug of abuse such as opioids and to ameliorate the withdrawal symptoms. Pseudoginsenoside-F(ll), a saponin contained in American ginseng, effectively attenuated methamphetamine-induced behavioral and nemochemical toxicides such as anxiety, depression, and memory deficits and alterations of monoamine contents in mice brain [148].
When the effects of total ginseng saponins were examined on presynaptic nicotine-induced dopamine (DA) release in Are striatum of freely moving rats using in vivo microdialysis technique and on the in vitro and in vivo binding of [3H]raclopride to DA D2 receptors, inhibition of nicotine-induced DA release and D2 receptor binding was observed along with behavioral inhibition of
nicotine-induced sensitization [149]. Similar results were observed with repeated cocaine administration. Pretreatment with GTS before the daily intraperitoneal injections of cocaine (15 mg/kg,) significantly inhibited die repeated cocaine-induced behavioral sensitization as well as die c-Fos expression in the core and shell of nucleus accumbens. In addition, pretreatment with GTS significantly decreased the repeated cocaine-induced increase in DA release in the nucleus accumbens as determined by in vivo microdialysis [150]. Similarly, Using real-time measurements of the extracellular DA concentrations by real-time fast-scan cyclic voltammetry in slices of rat brain nucleus accumbens, it has been demonstrated tiiat со-treatment of GTS inhibited the release enhancement of DA and subsequently prevented the rebound increase during acute withdrawal of cocaine without affecting die cocaine-mediated DA uptake [151].
In addition, morphine withdrawal-induced anxiety and depression-like behavior were effectively inhibited by wild ginseng extracts by modulating hypothalamic expression of CRF and NPY [133]. Moreover, die same group of researchers have reported diat wild ginseng extracts effectively attenuated morphine withdrawal-induced behavioral sensitization along with modulation of c-Fos expression in nucleus accumbens and TH expression in ventral tegmental area suggesting the possible involvement of ginseng in die modulation of dopaminergic nervous system activity during morphine withdrawal [152].
The signaling pathway leading to the decreased DA release and die neurobiological targets of ginseng mediating the up/down-regulation of proteins involved in die addiction subsequent to the withdrawal of drug of abuse remains to be determined, although regulation of intracellular ion influx including calcium seems to be one of flic plausible targets.
OTHER PSYCHIATRIC DISORDERS
In a rat model of epileptic seizure, pentylenetetrazole was injected intraperitoneally at the dose of 30 mg/kg, on alternate days to obtain generalized tonic-clonic convulsions in rats and pretreatment of P ginseng protected the rats from epileptic seizure [153]. Based on the fast inhibition of ginseng total saponins and ginsenoside Rg3 on NMDA receptor-mediated |Ca2 | in cultured hippocampal neurons, Kim and Rliim [154] determined that ginseng total saponins and ginsenoside Rg3 inhibited Mg2+ free-induced increase of |Ca2 | and spontaneous |Ca2 I oscillations in cultured rat hippocampal neurons suggesting ginseng may be effective in correcting the epileptiform discharge induced by excitatory/inhibitory imbalance. In an experiments using three different chemical seizure inducing agents, i.e., pentylenetetrazole, kainic acid, and pilocarpine, American ginseng provided protective effects against seizure [155]. Albeit the root preparation or leaves/stem preparation provided a marginal range of protection, the partially purified extracts that concentrate Rb ginsenosides (Rb extract) had a dosedependent anticonvulsant effect in all three models of chemically induced seizures. Rb extracts also reduced neuronal stress induced by kainic acid as evidenced by the reduced immunohistochemical signal against heat shock protein 72 [155]. The same group of researchers expanded their efforts to identify active components in ginseng extracts and concluded that flic mixtures of purified Rbl and Rb3 provided most robust anti-epileptic activity [156]. Interestingly, no single ginsenoside provided enough anti-epileptic and neuroprotective effects, which suggest that the combination of different ginsenosides is needed for optimal results [156].
Ginseng has been suggested to be effective in schizo- pherinic patients. In a 4 wk double-blind, placebo- controlled study using HT1001, a proprietary North American ginseng extract, unproved working memory in schizophrenic patients [157]. In addition to flic verbal and visual working memory improvement, HT101 reduced extrapyramidal symptoms after 4 wk treatment suggesting HT1001 might be effective in treating schizophrenia as well as to reduce the side effects of currently available medications [157]. Similarly, P. quinquefolius showed various range of neurological effects in ketamine-induced experimental psychosis model in mice without severe extra-pyramidal side effects and catalepsy [158]. Increased glutamate contents and decreased DA and 5-HT contents are evident along with increased acetylcholine esterase and nitrate level, which may impose flic need for further study.
NEURODEVELOPMENTAL DISORDERS
A recent drastic increase in prevalence of several neu- rodevelopmental diseases including ADHD and autism spectrum disorder (ASD) along with the relative lack of safe and efficient therapeutic agents in this field albeit flic wide spread use of stimulant medication in the case of ADHD, necessitates the investigation whether ginseng has therapeutic potential in this group of diseases. Surprisingly, only a small number of investigations have been reported so far, mostly focused on ADHD. Using a combinatorial herbal medicine consists of American ginseng and Ginko biloba, 36 children ranging in age from 3 to 17 yr with ADHD was enrolled in open-label clinical study [159]. After 4 wk treatment of tire herbal mixture, parents completed revised version of Conners' Parent Rating Scale and the authors reported that 44% to 74% of patients showed some kind of improvement either in social problem attributes or DSM-IV hyperactive-impulsive attribute [159]. The study hampers with the small number of subjects, and even with the initially promising results, no follow up study has been reported so far. Up to now, contradictory results have been reported about tire possible effects of ginseng on hyperactive phenotype in experimental animals. Chronic oral administration of ginseng slightly decreased the activity in open field test while it did not produce any significant difference against hyperactivity induced by amphetamine [136], while others reported the attenuation of behavioral sensitization induced by chemical stimulants [138,139,142]. More recently, it has been also suggested that three ADHD inattentive type patients showed improvements in their hyperactivity and inattention symptoms after taking P ginseng as judged by Conners' parent ratings [160]. Obviously, clinical studies with more number of patients as well as animal studies using relevant models and behavioral paradigms might be needed in this field [161].
ASD is a neurodevelopmental disorder characterized by tire impairment in communication, social interaction, and repetitive behavior. Although it has been suggested drat P. ginseng may have beneficial effects in the treatment of ASD patients in a preliminary study with human patients [162], no definite and elaborate study has been conducted in either animal and human studies. Interestingly, one recent article suggested that the active acidic polysaccharide portion of P ginseng increased social engagement both in time and frequency in normal mice. The reason for increased engagement was majorly attributed to the increased non-aggressive engagement in mice which is interpreted being related to the anti-depressant effects of ginseng [163]. Combined with the fact that ginseng may have efficacy in seizure, anxiety and schizophrenia, investigating whether ginseng might have therapeutic effects in this devastating disorder would be helpful to devise tools for intervention against the otherwise non-curable disorder.
CONCLUSION
Ginseng and ginsenosides affect various aspects of neurodevelopmental disorder including AD and PD as well as nemopsychiatric disorders and even neurodevelopmental disorder. Even with the vast array of researches conducted during last two decades, it still needs elaboration on the molecular and cellular mechanism of action. Even in studies showing the effects on therapeutic targets, the causal relationship of the target modulation is not clear. Obviously, investigations using more pharmacological model systems as well as genetic model systems would be essential to unravel the therapeutic efficacy as well as molecular mechanisms. Recent progress in high-throughput measures to study biological processes such as omics technology would be beneficial in tire field of ginseng research as well. Unresolved questions such as tire obvious therapeutic efficacy even with tire poor kinetic profiles of some ginsenosides after oral administration, are being approached in several ways, for example, demonstrating the therapeutic efficacy of ginsenoside metabolites such as compound К and devising new way of administration including intranasal administration. It is increasingly evident that not a single molecular target can explain all the pathophyiological features of a given neurological diseases. In many cases such as AD and ischemia, hope to develop a therapeutic agent based on a single molecular targets is almost coming to an end unless tire ultimate new target is developed and available for those invincible disease. In this regard, the fact that ginseng affects seemingly a multiple number of targets regulating synaptic plasticity, neurogenesis, neuroprotection, neural transmission, and much more, is worthy of special attention. Finally, the comparison of pharmacological and therapeutic effects of different ginseng preparation needs standardization of preparation otherwise there's no way to improve our understanding of the differential effects of specific ginseng preparation on particular neurological disorders. With the unceasing efforts of the existing and forthcoming investigators in this field, future will tell us whether ginseng will provide effective tools to combat with AD, PD, ischemia and other neurological condition or disorders.
ACKNOWLEDGEMENTS
This work was supported by Konkuk University in 2012.
REFERENCES
- Lu G, Zhou Q, Sun S, Leung KS, Zhang H, Zhao Z. Differentiation of Asian ginseng, American ginseng and Notoginseng by Fourier transform infrared spectroscopy combined with two-dimensional correlation infrared spectroscopy. J Mol Struct 2008;883-884:91-98. https://doi.org/10.1016/j.molstruc.2007.12.008
- Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of ginseng saponins on responses induced by various receptor stimuli. Eur J Pharmacol 1999;369:23-32. https://doi.org/10.1016/S0014-2999(99)00043-6
- Sung H, Jung YS, Cho YK. Beneficial effects of a combination of Korean red ginseng and highly active antiretroviral therapy in human immunodeficiency virus type 1-infected patients. Clin Vaccine Immunol 2009;16:1127-1131. https://doi.org/10.1128/CVI.00013-09
- Radad K, Moldzio R, Rausch WD. Ginsenosides and their CNS targets. CNS Neurosci Ther 2011;17:761-768. https://doi.org/10.1111/j.1755-5949.2010.00208.x
- Jesky R, Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments? An integrative look at memory, dementia and the promising therapeutics of traditional chinese medicines. Phytother Res 2011;25:1105-1118. https://doi.org/10.1002/ptr.3388
- Radad K, Gille G, Liu L, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 2006;100:175-186. https://doi.org/10.1254/jphs.CRJ05010X
- Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Axonal and dendritic extension by protopanaxadiol-type saponins from ginseng drugs in SK-N-SH cells. Jpn J Pharmacol 2002;90:254-262. https://doi.org/10.1254/jjp.90.254
- Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Abeta(25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology 2004;29:860-868. https://doi.org/10.1038/sj.npp.1300388
- Sugaya A, Yuzurihara M, Tsuda T, Yasuda K, Kajiwara K, Sugaya E. Proliferative effect of ginseng saponin on neurite extension of primary cultured neurons of the rat cerebral cortex. J Ethnopharmacol 1988;22:173-181. https://doi.org/10.1016/0378-8741(88)90125-0
- Nishiyama N, Cho SI, Kitagawa I, Saito H. Malonylginsenoside Rb1 potentiates nerve growth factor (NGF)-induced neurite outgrowth of cultured chick embryonic dorsal root ganglia. Biol Pharm Bull 1994;17:509-513. https://doi.org/10.1248/bpb.17.509
- Wang XY, Zhang JT. Effect of ginsenoside Rb1 on long-term potentiation in the dentate gyrus of anaesthetized rats. J Asian Nat Prod Res 2003;5:1-4. https://doi.org/10.1080/10286020290029009
- Kurimoto H, Nishijo H, Uwano T, Yamaguchi H, Zhong YM, Kawanishi K, Ono T. Effects of nonsaponin fraction of red ginseng on learning deficits in aged rats. Physiol Behav 2004;82:345-355. https://doi.org/10.1016/j.physbeh.2004.04.001
- Qi D, Zhu Y, Wen L, Liu Q, Qiao H. Ginsenoside Rg1 restores the impairment of learning induced by chronic morphine administration in rats. J Psychopharmacol 2009;23:74-83. https://doi.org/10.1177/0269881107082950
- Bae MY, Cho JH, Choi IS, Park HM, Lee MG, Kim DH, Jang IS. Compound K, a metabolite of ginsenosides, facilitates spontaneous GABA release onto CA3 pyramidal neurons. J Neurochem 2010;114:1085-1096.
- Jiang B, Xiong Z, Yang J, Wang W, Wang Y, Hu ZL, Wang F, Chen JG. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol 2012;166:1872-1887. https://doi.org/10.1111/j.1476-5381.2012.01902.x
- Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin 2005;26:143-149. https://doi.org/10.1111/j.1745-7254.2005.00034.x
- Wang L, Kisaalita WS. Administration of BDNF/ginsenosides combination enhanced synaptic development in human neural stem cells. J Neurosci Methods 2011;194:274-282. https://doi.org/10.1016/j.jneumeth.2010.10.025
- Li N, Liu B, Dluzen DE, Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol 2007;111:458-463. https://doi.org/10.1016/j.jep.2006.12.015
- Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev 2007;13:381-404.
- Tu LH, Ma J, Liu HP, Wang RR, Luo J. The neuroprotective effects of ginsenosides on calcineurin activity and tau phosphorylation in SY5Y cells. Cell Mol Neurobiol 2009;29:1257-1264. https://doi.org/10.1007/s10571-009-9421-3
- Hu SQ, Yu HM, Liu TS, Yang DJ, Chen XZ, He CJ. Neuroprotective effects of water extracts of American ginseng on SH-SY5Y cells apoptosis induced by Abeta25-35. Zhong Yao Cai 2008;31:1373-1377.
- Xie X, Wang HT, Li CL, Gao XH, Ding JL, Zhao HH, Lu YL. Ginsenoside Rb1 protects PC12 cells against β-amyloid-induced cell injury. Mol Med Report 2010;3:635-639.
- Wang YH, Du GH. Ginsenoside Rg1 inhibits beta-secretase activity in vitro and protects against Abeta-induced cytotoxicity in PC12 cells. J Asian Nat Prod Res 2009;11:604-612. https://doi.org/10.1080/10286020902843152
- Shieh PC, Tsao CW, Li JS, Wu HT, Wen YJ, Kou DH, Cheng JT. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci Lett 2008;434:1-5. https://doi.org/10.1016/j.neulet.2007.12.032
- Lin WM, Zhang YM, Moldzio R, Rausch WD. Ginsenoside Rd attenuates neuroinflammation of dopaminergic cells in culture. J Neural Transm Suppl 2007;(72):105-112.
- Shen T, Lee J, Park MH, Lee YG, Rho HS, Kwak YS, Rhee MH, Park YC, Cho JY: Ginsenoside Rp1, a ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKKb-mediated NF-kB pathway in HEK293 cells. J Ginseng Res 2011;35:200-208. https://doi.org/10.5142/jgr.2011.35.2.200
- Wu CF, Bi XL, Yang JY, Zhan JY, Dong YX, Wang JH, Wang JM, Zhang R, Li X. Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol 2007;7:313-320. https://doi.org/10.1016/j.intimp.2006.04.021
- Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH, Kim DH. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial cells. Planta Med 2006;72:627-633. https://doi.org/10.1055/s-2006-931563
- Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett 2011;487:70-72. https://doi.org/10.1016/j.neulet.2010.09.076
- Park JS, Shin JA, Jung JS, Hyun JW, Van Le TK, Kim DH, Park EM, Kim HS. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther 2012;341:59-67. https://doi.org/10.1124/jpet.111.189035
- He W, Zhu Z. Effect of Panax notoginseng saponins on intercellular adhesion molecule-1 expression and neutrophil infiltration in cerebral infarction tissue of rats. Zhong Yao Cai 2005;28:403-405.
- Joo SS, Won TJ, Lee DI. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vivo. Planta Med 2005;71:476-481. https://doi.org/10.1055/s-2005-864145
- Zhao H, Li Q, Zhang Z, Pei X, Wang J, Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res 2009;1256:111-122. https://doi.org/10.1016/j.brainres.2008.12.031
- Jung JS, Shin JA, Park EM, Lee JE, Kang YS, Min SW, Kim DH, Hyun JW, Shin CY, Kim HS. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem 2010;115:1668-1680. https://doi.org/10.1111/j.1471-4159.2010.07075.x
- Song SB, Tung NH, Quang TH, Ngan NT, Kim KE, Kim YH: Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by dammarane-type saponins from Panax ginseng leaves. J Ginseng Res 2012; 36:146-152. https://doi.org/10.5142/jgr.2012.36.2.146
- Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. FASEB J 2006;20:1269-1271. https://doi.org/10.1096/fj.05-5530fje
- Choi RC, Zhu JT, Leung KW, Chu GK, Xie HQ, Chen VP, Zheng KY, Lau DT, Dong TT, Chow PC et al. A flavonol glycoside, isolated from roots of Panax notoginseng, reduces amyloid-beta-induced neurotoxicity in cultured neurons: signaling transduction and drug development for Alzheimer’s disease. J Alzheimers Dis 2010;19:795-811. https://doi.org/10.3233/JAD-2010-1293
- Chen LM, Lin ZY, Zhu YG, Lin N, Zhang J, Pan XD, Chen XC. Ginsenoside Rg1 attenuates β-amyloid generation via suppressing PPARγ-regulated BACE1 activity in N2a-APP695 cells. Eur J Pharmacol 2012;675:15-21. https://doi.org/10.1016/j.ejphar.2011.11.039
- Fang F, Chen X, Huang T, Lue LF, Luddy JS, Yan SS. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim Biophys Acta 2012;1822:286-292. https://doi.org/10.1016/j.bbadis.2011.10.004
- Shi C, Na N, Zhu X, Xu J. Estrogenic effect of ginsenoside Rg1 on APP processing in post-menopausal platelets. Platelets 2012; Epub ahead of print.
- Shi C, Zheng DD, Fang L, Wu F, Kwong WH, Xu J. Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim Biophys Acta 2012;1820:453-460. https://doi.org/10.1016/j.bbagen.2011.12.005
- Joo SS, Lee DI. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: enhancement of type A Macrophage Scavenger Receptor expression. Arch Pharm Res 2005;28:1164-1169. https://doi.org/10.1007/BF02972981
- Yang L, Hao J, Zhang J, Xia W, Dong X, Hu X, Kong F, Cui X. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J Pharm Pharmacol 2009;61:375-380. https://doi.org/10.1211/jpp.61.03.0013
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science 2001;292:1550-1552. https://doi.org/10.1126/science.1059946
- Xie YH, Chen XC, Zhang J, Huang TW, Song JQ, Fang YX, Pan XD, Lin ZY. Ginsenoside Rb1 attenuates beta-amyloid peptide(25-35)-induced hyperphosphorylation of tau protein through CDK5 signal pathway. Yao Xue Xue Bao 2007;42:828-832.
- Zhao R, Zhang Z, Song Y, Wang D, Qi J, Wen S. Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3β pathway in ginsenoside Rb1's attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. J Ethnopharmacol 2011;133:1109-1116. https://doi.org/10.1016/j.jep.2010.11.054
- Li X, Liu Y, Zhang X, Yuan H, Quan Q. Effect of ginsenoside Rg1 on expressions of phosphory protein tau and N-methyl-D-aspartate receptor subunits NR1 and NR2B in rat brain slice model of Alzheimer's disease. Zhongguo Zhong Yao Za Zhi 2010;35:3339-3343.
- Li L, Liu J, Yan X, Qin K, Shi M, Lin T, Zhu Y, Kang T, Zhao G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J Ethnopharmacol 2011;138:135-141. https://doi.org/10.1016/j.jep.2011.08.068
- Hao W, Xing-Jun W, Yong-Yao C, Liang Z, Yang L, Hong-Zhuan C. Up-regulation of M1 muscarinic recep tors expressed in CHOm1 cells by panaxynol via cAMP pathway. Neurosci Lett 2005;383:121-126 https://doi.org/10.1016/j.neulet.2005.03.062
- Xue JF, Liu ZJ, Hu JF, Chen H, Zhang JT, Chen NH. Ginsenoside Rb1 promotes neurotransmitter release by modulating phosphorylation of synapsins through a cAMP-dependent protein kinase pathway. Brain Res 2006;1106:91-98. https://doi.org/10.1016/j.brainres.2006.05.106
- Benishin CG, Lee R, Wang LC, Liu HJ. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology 1991;42:223-229. https://doi.org/10.1159/000138801
- Benishin CG. Actions of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem Int 1992;21:1-5. https://doi.org/10.1016/0197-0186(92)90061-U
- Salim KN, McEwen BS, Chao HM. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Brain Res Mol Brain Res 1997;47:177-182. https://doi.org/10.1016/S0169-328X(97)00042-9
- Zhang JT, Qu ZW, Liu Y, Deng HL. Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chin Med J (Engl) 1990;103:932-938.
- Lee TF, Shiao YJ, Chen CF, Wang LC. Effect of ginseng saponins on beta-amyloid-suppressed acetylcholine release from rat hippocampal slices. Planta Med 2001;67:634-637. https://doi.org/10.1055/s-2001-17366
- Lee NH, Son CG. Systematic review of randomized controlled trials evaluating the efficacy and safety of ginseng. J Acupunct Meridian Stud 2011;4:85-97. https://doi.org/10.1016/S2005-2901(11)60013-7
- Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, Shim JY, Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur J Neurol 2008;15:865-868. https://doi.org/10.1111/j.1468-1331.2008.02157.x
- Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord 2008;22:222-226. https://doi.org/10.1097/WAD.0b013e31816c92e6
- Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol 2003;473:1-7. https://doi.org/10.1016/S0014-2999(03)01945-9
- Liu Q, Kou JP, Yu BY. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int 2011;58:119-125. https://doi.org/10.1016/j.neuint.2010.11.004
- Radad K, Gille G, Moldzio R, Saito H, Ishige K, Rausch WD. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J Neural Transm 2004;111:37-45. https://doi.org/10.1007/s00702-003-0063-1
- Chen XC, Zhou YC, Chen Y, Zhu YG, Fang F, Chen LM. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin 2005;26:56-62. https://doi.org/10.1111/j.1745-7254.2005.00019.x
- Wang J, Xu HM, Yang HD, Du XX, Jiang H, Xie JX. Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins. Neurochem Int 2009;54:43-48. https://doi.org/10.1016/j.neuint.2008.10.003
- Xu H, Jiang H, Wang J, Xie J. Rg1 protects iron-induced neurotoxicity through antioxidant and iron regulatory proteins in 6-OHDA-treated MES23.5 cells. J Cell Biochem 2010;111:1537-1545. https://doi.org/10.1002/jcb.22885
- Xu H, Jiang H, Wang J, Xie J. Rg1 protects the MPP+-treated MES23.5 cells via attenuating DMT1 up-regulation and cellular iron uptake. Neuropharmacology 2010;58:488-494. https://doi.org/10.1016/j.neuropharm.2009.09.002
- Luo FC, Wang SD, Li K, Nakamura H, Yodoi J, Bai J. Panaxatriol saponins extracted from Panax notoginseng induces thioredoxin-1 and prevents 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. J Ethnopharmacol 2010;127:419-423. https://doi.org/10.1016/j.jep.2009.10.023
- Luo FC, Wang SD, Qi L, Song JY, Lv T, Bai J. Protective effect of panaxatriol saponins extracted from Panax notoginseng against MPTP-induced neurotoxicity in vivo. J Ethnopharmacol 2011;133:448-453. https://doi.org/10.1016/j.jep.2010.10.017
- Leung KW, Yung KK, Mak NK, Chan YS, Fan TP, Wong RN. Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology 2007;52:827-835. https://doi.org/10.1016/j.neuropharm.2006.10.001
- Ge KL, Chen WF, Xie JX, Wong MS. Ginsenoside Rg1 protects against 6-OHDA-induced toxicity in MES23.5 cells via Akt and ERK signaling pathways. J Ethnopharmacol 2010;127:118-123. https://doi.org/10.1016/j.jep.2009.09.038
- Gao QG, Chen WF, Xie JX, Wong MS. Ginsenoside Rg1 protects against 6-OHDA-induced neurotoxicity in neuroblastoma SK-N-SH cells via IGF-I receptor and estrogen receptor pathways. J Neurochem 2009;109:1338-1347. https://doi.org/10.1111/j.1471-4159.2009.06051.x
- Xu L, Chen WF, Wong MS. Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson's disease through the IGF-I receptor signalling pathway. Br J Pharmacol 2009;158:738-748. https://doi.org/10.1111/j.1476-5381.2009.00361.x
- Beamer CA, Shepherd DM. Inhibition of TLR ligand- and interferon gamma-induced murine microglial activation by Panax notoginseng. J Neuroimmune Pharmacol 2012;7:465-476. https://doi.org/10.1007/s11481-011-9333-0
- Van Kampen J, Robertson H, Hagg T, Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson's disease. Exp Neurol 2003;184:521-529. https://doi.org/10.1016/j.expneurol.2003.08.002
- Rudakewich M, Ba F, Benishin CG. Neurotrophic and neuroprotective actions of ginsenosides Rb(1) and Rg(1). Planta Med 2001;67:533-537. https://doi.org/10.1055/s-2001-16488
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron 2010;66:646-661. https://doi.org/10.1016/j.neuron.2010.04.034
- Wu J, Jeong HK, Bulin SE, Kwon SW, Park JH, Bezprozvanny I. Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J Neurosci Res 2009;87:1904-1912. https://doi.org/10.1002/jnr.22017
- Jiang F, DeSilva S, Turnbull J. Beneficial effect of ginseng root in SOD-1 (G93A) transgenic mice. J Neurol Sci 2000;180:52-54. https://doi.org/10.1016/S0022-510X(00)00421-4
- Shah ZA, Gilani RA, Sharma P, Vohora SB. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol 2005;101:299-307. https://doi.org/10.1016/j.jep.2005.05.002
- Kim YO, Kim HJ, Kim GS, Park HG, Lim SJ, Seong NS, Ham YW, Lee SD, Jang KH, Jung KH et al. Panax ginseng protects against global ischemia injury in rat hippocampus. J Med Food 2009;12:71-76. https://doi.org/10.1089/jmf.2007.0614
- Chu GX, Chen X. Anti-lipid peroxidation and protection of ginsenosides against cerebral ischemia-reperfusion injuries in rats. Zhongguo Yao Li Xue Bao 1990;11:119-123.
- Wen TC, Yoshimura H, Matsuda S, Lim JH, Sakanaka M. Ginseng root prevents learning disability and neuronal loss in gerbils with 5-minute forebrain ischemia. Acta Neuropathol 1996;91:15-22.
- Wang J, Yang LJ, Zhou CM, Zhu HM, Zhang SM. Effects of Shenfu injection on hypoxic-ischemic brain damage: experiment with neonatal rats. Zhonghua Yi Xue Za Zhi 2006;86:2994-2997.
- Kim YC, Kim SR, Markelonis GJ, Oh TH. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J Neurosci Res 1998;53:426-432. https://doi.org/10.1002/(SICI)1097-4547(19980815)53:4<426::AID-JNR4>3.0.CO;2-8
- Ye R, Li N, Han J, Kong X, Cao R, Rao Z, Zhao G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res 2009;64:306-310. https://doi.org/10.1016/j.neures.2009.03.016
- Ye R, Kong X, Yang Q, Zhang Y, Han J, Zhao G. Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology 2011;61:815-824. https://doi.org/10.1016/j.neuropharm.2011.05.029
- Tian J, Zhang S, Li G, Liu Z, Xu B. 20(S)-ginsenoside Rg3, a neuroprotective agent, inhibits mitochondrial permeability transition pores in rat brain. Phytother Res 2009;23:486-491. https://doi.org/10.1002/ptr.2653
- Zhang B, Hata R, Zhu P, Sato K, Wen TC, Yang L, Fujita H, Mitsuda N, Tanaka J, Samukawa K et al. Prevention of ischemic neuronal death by intravenous infusion of a ginseng saponin, ginsenoside Rb(1), that upregulates Bcl-x(L) expression. J Cereb Blood Flow Metab 2006;26:708-721. https://doi.org/10.1038/sj.jcbfm.9600225
- Park EK, Choo MK, Oh JK, Ryu JH, Kim DH. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm Bull 2004;27:433-436. https://doi.org/10.1248/bpb.27.433
- Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M et al. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 2011;178:169-180.
- Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int 2011;58:391-398. https://doi.org/10.1016/j.neuint.2010.12.015
- Zhang Y, Zhou L, Zhang X, Bai J, Shi M, Zhao G. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci 2012;33:1125-1131. https://doi.org/10.1007/s10072-011-0916-6
- He W, Zhu Z, Liu J, Ye H, Zeng J, Huang X, Lai F. Study on therapeutic window of opportunity for Panax notoginseng saponins following focal cerebral ischemia/reperfusion injury in rats. Zhong Yao Cai 2004;27:25-27.
- Lee JS, Choi HS, Kang SW, Chung JH, Park HK, Ban JY, Kwon OY, Hong HP, Ko YG. Therapeutic effect of Korean red ginseng on inflammatory cytokines in rats with focal cerebral ischemia/reperfusion injury. Am J Chin Med 2011;39:83-94. https://doi.org/10.1142/S0192415X1100866X
- Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG. Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol 2009;121:412-418. https://doi.org/10.1016/j.jep.2008.10.042
- Lu T, Jiang Y, Zhou Z, Yue X, Wei N, Chen Z, Ma M, Xu G, Liu X. Intranasal ginsenoside Rb1 targets the brain and ameliorates cerebral ischemia/reperfusion injury in rats. Biol Pharm Bull 2011;34:1319-1324. https://doi.org/10.1248/bpb.34.1319
- Zhu J, Jiang Y, Wu L, Lu T, Xu G, Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience 2012;202:342-351. https://doi.org/10.1016/j.neuroscience.2011.11.070
- Park HJ, Shim HS, Kim KS, Shim I. The protective effect of black ginseng against transient focal ischemia-induced neuronal damage in rats. Korean J Physiol Pharmacol 2011;15:333-338. https://doi.org/10.4196/kjpp.2011.15.6.333
- Park SI, Jang DK, Han YM, Sunwoo YY, Park MS, Chung YA, Maeng LS, Im R, Kim MW, Jeun SS et al. Effect of combination therapy with sodium ozagrel and Panax ginseng on transient cerebral ischemia model in rats. J Biomed Biotechnol 2010;2010:893401.
- Sakanaka M, Zhu P, Zhang B, Wen TC, Cao F, Ma YJ, Samukawa K, Mitsuda N, Tanaka J, Kuramoto M et al. Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl-XL. J Neurotrauma 2007;24:1037-1054. https://doi.org/10.1089/neu.2006.0182
- Zhang B, Matsuda S, Tanaka J, Tateishi N, Maeda N, Wen TC, Peng H, Sakanaka M. Ginsenoside Rb(1) prevents image navigation disability, cortical infarction, and thalamic degeneration in rats with focal cerebral ischemia. J Stroke Cerebrovasc Dis 1998;7:1-9. https://doi.org/10.1016/S1052-3057(98)80015-3
- Son HY, Han HS, Jung HW, Park YK. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J Pharmacol Sci 2009;109:368-379. https://doi.org/10.1254/jphs.08197FP
- Zhang G, Liu A, Zhou Y, San X, Jin T, Jin Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol 2008;115:441-448. https://doi.org/10.1016/j.jep.2007.10.026
- Zhang YG, Liu TP. Influences of ginsenosides Rb1 and Rg1 on reversible focal brain ischemia in rats. Zhongguo Yao Li Xue Bao 1996;17:44-48.
- Zheng GQ, Cheng W, Wang Y, Wang XM, Zhao SZ, Zhou Y, Liu SJ, Wang XT. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol 2011;133:724-728. https://doi.org/10.1016/j.jep.2010.01.064
- Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res 1997;28:191-200. https://doi.org/10.1016/S0168-0102(97)00041-2
- Choi SR, Saji H, Iida Y, Magata Y, Yokoyama A. Ginseng pretreatment protects against transient global cerebral ischemia in the rat: measurement of local cerebral glucose utilization by [14C]deoxyglucose autoradiography. Biol Pharm Bull 1996;19:644-646. https://doi.org/10.1248/bpb.19.644
- Chuang CM, Hsieh CL, Lin HY, Lin JG. Panax notoginseng Burk attenuates impairment of learning and memory functions and increases ED1, BDNF and beta-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats. Am J Chin Med 2008;36:685-693. https://doi.org/10.1142/S0192415X08006156
- Jiang S, Miao B, Song X, Jiang Z. Inactivation of GABA(A) receptor reduces ginsenoside Rb3 neuroprotection in mouse hippocampal slices after oxygen-glucose deprivation. J Ethnopharmacol 2011;133:914-916. https://doi.org/10.1016/j.jep.2010.10.030
- Park H, Kim H, Ha E, Yoon S, Kim MJ, Hong M, Leem KH, Hong SJ, Yang J, Chung JH. Panax ginseng increases hypoxia-induced down-regulated cellular response related genes in human neuroblastoma cells, SK-N-MC. Neurol Res 2007;29 Suppl 1:S78-S87.
- Si YC, Zhang JP, Xie CE, Zhang LJ, Jiang XN. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med 2011;39:999-1013. https://doi.org/10.1142/S0192415X11009366
- Zhu JR, Tao YF, Lou S, Wu ZM. Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells. Acta Pharmacol Sin 2010;31:273-280. https://doi.org/10.1038/aps.2010.9
- Siddique MS, Eddeb F, Mantle D, Mendelow AD. Extracts of Ginkgo biloba and Panax ginseng protect brain proteins from free radical induced oxidative damage in vitro. Acta Neurochir Suppl 2000;76:87-90.
- Choi SH, Shin TJ, Lee BH, Hwang SH, Lee SM, Lee BC, Park CS, Ha TS, Nah SY. Ginsenoside Rg3 enhances large conductance Ca2+-activated potassium channel currents: a role of Tyr360 residue. Mol Cells 2011;31:133-140. https://doi.org/10.1007/s10059-011-0017-7
- Liu D, Li B, Liu Y, Attele AS, Kyle JW, Yuan CS. Voltage-dependent inhibition of brain Na(+) channels by American ginseng. Eur J Pharmacol 2001;413:47-54. https://doi.org/10.1016/S0014-2999(01)00735-X
- Jang S, Ryu JH, Kim DH, Oh S. Changes of [3H]MK-801, [3H]muscimol and [3H]flunitrazepam binding in rat brain by the prolonged ventricular infusion of transformed ginsenosides. Neurochem Res 2004;29:2257-2266. https://doi.org/10.1007/s11064-004-7034-2
- Wang C, Li YZ, Wang XR, Lu ZR, Shi DZ, Liu XH. Panax quinquefolium saponins reduce myocardial hypoxia-reoxygenation injury by inhibiting excessive endoplasmic reticulum stress. Shock 2012;37:228-233. https://doi.org/10.1097/SHK.0b013e31823f15c4
- Chan LS, Yue PY, Mak NK, Wong RN. Role of microRNA-214 in ginsenoside-Rg1-induced angiogenesis. Eur J Pharm Sci 2009;38:370-377. https://doi.org/10.1016/j.ejps.2009.08.008
- Yue PY, Wong DY, Ha WY, Fung MC, Mak NK, Yeung HW, Leung HW, Chan K, Liu L, Fan TP et al. Elucidation of the mechanisms underlying the angiogenic effects of ginsenoside Rg(1) in vivo and in vitro. Angiogenesis 2005;8:205-216. https://doi.org/10.1007/s10456-005-9000-2
- Chen X, Salwinski S, Lee TJ. Extracts of Ginkgo biloba and ginsenosides exert cerebral vasorelaxation via a nitric oxide pathway. Clin Exp Pharmacol Physiol 1997;24:958-959. https://doi.org/10.1111/j.1440-1681.1997.tb02727.x
- Leung KW, Ng HM, Tang MK, Wong CC, Wong RN, Wong AS. Ginsenoside-Rg1 mediates a hypoxia-independent upregulation of hypoxia-inducible factor-1α to promote angiogenesis. Angiogenesis 2011;14:515-522. https://doi.org/10.1007/s10456-011-9235-z
- Wang Z, Li M, Wu WK, Tan HM, Geng DF. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther 2008;22:443-452. https://doi.org/10.1007/s10557-008-6129-4
- Yue QX, Xie FB, Song XY, Wu WY, Jiang BH, Guan SH, Yang M, Liu X, Guo DA. Proteomic studies on protective effects of salvianolic acids, notoginsengnosides and combination of salvianolic acids and notoginsengnosides against cardiac ischemic-reperfusion injury. J Ethnopharmacol 2012;141:659-667. https://doi.org/10.1016/j.jep.2011.08.044
- Chen X, Zhou M, Li Q, Yang J, Zhang Y, Zhang D, Kong S, Zhou D, He L. Sanchi for acute ischaemic stroke. Cochrane Database Syst Rev 2008;(4):CD006305.
- He L, Chen X, Zhou M, Zhang D, Yang J, Yang M, Zhou D. Radix/rhizome notoginseng extract (sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine 2011;18:437-442. https://doi.org/10.1016/j.phymed.2010.10.004
- Xuejiang W, Magara T, Konishi T. Prevention and repair of cerebral ischemia-reperfusion injury by Chinese herbal medicine, shengmai san, in rats. Free Radic Res 1999;31:449-455. https://doi.org/10.1080/10715769900301011
- Zheng M, Qu L, Lou Y. Effects of icariin combined with Panax notoginseng saponins on ischemia reperfusion-induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat. Phytother Res 2008;22:597-604. https://doi.org/10.1002/ptr.2276
- Hartley DE, Elsabagh S, File SE. Gincosan (a combination of Ginkgo biloba and Panax ginseng): the effects on mood and cognition of 6 and 12 weeks’ treatment in post-menopausal women. Nutr Neurosci 2004;7:325-333. https://doi.org/10.1080/10284150400015557
- Kuribara H, Tomioka H, Takahashi R, Onozato K, Murohashi N, Numajiri T, Iwata H, Koya S. An antidepressant effect of Sho-ju-sen, a Japanese herbal medicine, assessed by learned helplessness model in mice. Phytother Res 2004;18:173-176. https://doi.org/10.1002/ptr.1412
- Dang H, Sun L, Liu X, Peng B, Wang Q, Jia W, Chen Y, Pan A, Xiao P. Preventive action of Kai Xin San aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp Biol Med (Maywood) 2009;234:785-793. https://doi.org/10.3181/0812-RM-354
- Dang H, Chen Y, Liu X, Wang Q, Wang L, Jia W, Wang Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:1417-1424. https://doi.org/10.1016/j.pnpbp.2009.07.020
- Chatterjee M, Verma P, Palit G. Comparative evaluation of Bacopa monniera and Panax quniquefolium in experimental anxiety and depressive models in mice. Indian J Exp Biol 2010;48:306-313.
- Kim NH, Kim KY, Jeong HJ, Kim HM. Antidepressant-like effect of altered Korean red ginseng in mice. Behav Med 2011;37:42-46. https://doi.org/10.1080/08964289.2011.566591
- Lee B, Kim H, Shim I, Lee H, Hahm DH. Wild ginseng attenuates anxiety- and depression-like behaviors during morphine withdrawal. J Microbiol Biotechnol 2011;21:1088-1096. https://doi.org/10.4014/jmb.1106.06027
- Liu L, Luo Y, Zhang R, Guo J. Effects of ginsenosides on hypothalamic-pituitary-adrenal function and brain-derived neurotrophic factor in rats exposed to chronic unpredictable mild stress. Zhongguo Zhong Yao Za Zhi 2011;36:1342-1347.
- Wang Z, Dai J, Chen L, Huang Y, Zhao Y. Preventive action of Panax ginseng roots in hypercortisolism-induced Impairment of hippocampal neurons in male C57BL/6N mice. Phytother Res 2011;25:1242-1245. https://doi.org/10.1002/ptr.3389
- Yamada N, Araki H, Yoshimura H. Identification of antidepressant-like ingredients in ginseng root (Panax ginseng C.A. Meyer) using a menopausal depressive-like state in female mice: participation of 5-HT2A receptors. Psychopharmacology (Berl) 2011;216:589-599. https://doi.org/10.1007/s00213-011-2252-1
- Tode T, Kikuchi Y, Hirata J, Kita T, Nakata H, Nagata I. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet 1999;67:169-174. https://doi.org/10.1016/S0020-7292(99)00168-X
- Xu C, Teng J, Chen W, Ge Q, Yang Z, Yu C, Yang Z, Jia W. 20(S)-protopanaxadiol, an active ginseng metabolite, exhibits strong antidepressant-like effects in animal tests. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:1402-1411. https://doi.org/10.1016/j.pnpbp.2010.07.010
- Kang A, Hao H, Zheng X, Liang Y, Xie Y, Xie T, Dai C, Zhao Q, Wu X, Xie L et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J Neuroinflammation 2011;8:100. https://doi.org/10.1186/1742-2094-8-100
- Bhattacharya SK, Mitra SK. Anxiolytic activity of Panax ginseng roots: an experimental study. J Ethnopharmacol 1991;34:87-92. https://doi.org/10.1016/0378-8741(91)90193-H
- Churchill JD, Gerson JL, Hinton KA, Mifek JL, Walter MJ, Winslow CL, Deyo RA. The nootropic properties of ginseng saponin Rb1 are linked to effects on anxiety. Integr Physiol Behav Sci 2002;37:178-187. https://doi.org/10.1007/BF02734180
- Cha HY, Park JH, Hong JT, Yoo HS, Song S, Hwang BY, Eun JS, Oh KW. Anxiolytic-like effects of ginsenosides on the elevated plus-maze model in mice. Biol Pharm Bull 2005;28:1621-1625. https://doi.org/10.1248/bpb.28.1621
- Carr MN, Bekku N, Yoshimura H. Identification of anxiolytic ingredients in ginseng root using the elevated plus-maze test in mice. Eur J Pharmacol 2006;531:160-165. https://doi.org/10.1016/j.ejphar.2005.12.014
- Kim TW, Choi HJ, Kim NJ, Kim DH. Anxiolytic-like effects of ginsenosides Rg3 and Rh2 from red ginseng in the elevated plus-maze model. Planta Med 2009;75:836-839. https://doi.org/10.1055/s-0029-1185402
- Wei XY, Yang JY, Wang JH, Wu CF. Anxiolytic effect of saponins from Panax quinquefolium in mice. J Ethnopharmacol 2007;111:613-618. https://doi.org/10.1016/j.jep.2007.01.009
- Einat H. Chronic oral administration of ginseng extract results in behavioral change but has no effects in mice models of affective and anxiety disorders. Phytother Res 2007;21:62-66. https://doi.org/10.1002/ptr.2024
- Steimer T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin Neurosci 2011;13:495-506.
- Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, Yang JY. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol Biochem Behav 2003;76:103-109. https://doi.org/10.1016/S0091-3057(03)00215-6
- Kim SE, Shim I, Chung JK, Lee MC. Effect of ginseng saponins on enhanced dopaminergic transmission and locomotor hyperactivity induced by nicotine. Neuropsychopharmacology 2006;31:1714-1721. https://doi.org/10.1038/sj.npp.1300945
- Lee B, Yang CH, Hahm DH, Lee HJ, Han SM, Kim KS, Shim I. Inhibitory effects of ginseng total saponins on behavioral sensitization and dopamine release induced by cocaine. Biol Pharm Bull 2008;31:436-441. https://doi.org/10.1248/bpb.31.436
- Nah SY, Bhatia KS, Lyles J, Ellinwood EH, Lee TH. Effects of ginseng saponin on acute cocaine-induced alterations in evoked dopamine release and uptake in rat brain nucleus accumbens. Brain Res 2009;1248:184-190. https://doi.org/10.1016/j.brainres.2008.10.064
- Lee B, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Wild ginseng attenuates repeated morphine-induced behavioral sensitization in rats. J Microbiol Biotechnol 2011;21:757-765. https://doi.org/10.4014/jmb.1103.03016
- Gupta YK, Sharma M, Chaudhary G. Antiepileptic activity of Panax ginseng against pentylenetetrazole induced kindling in rats. Indian J Physiol Pharmacol 2001;45:502-506.
- Kim S, Rhim H. Ginsenosides inhibit NMDA receptor-mediated epileptic discharges in cultured hippocampal neurons. Arch Pharm Res 2004;27:524-530. https://doi.org/10.1007/BF02980126
- Lian XY, Zhang ZZ, Stringer JL. Anticonvulsant activity of ginseng on seizures induced by chemical convulsants. Epilepsia 2005;46:15-22.
- Lian XY, Zhang Z, Stringer JL. Anticonvulsant and neuroprotective effects of ginsenosides in rats. Epilepsy Res 2006;70:244-256. https://doi.org/10.1016/j.eplepsyres.2006.05.010
- Chen EY, Hui CL. HT1001, a proprietary North American ginseng extract, improves working memory in schizophrenia: a double-blind, placebo-controlled study. Phytother Res 2012;26:1166-1172. https://doi.org/10.1002/ptr.3700
- Chatterjee M, Singh S, Kumari R, Verma AK, Palit G. Evaluation of the antipsychotic potential of Panax quinquefolium in ketamine induced experimental psychosis model in mice. Neurochem Res 2012;37:759-770. https://doi.org/10.1007/s11064-011-0670-4
- Lyon MR, Cline JC, Totosy de Zepetnek J, Shan JJ, Pang P, Benishin C. Effect of the herbal extract combination Panax quinquefolium and Ginkgo biloba on attention-deficit hyperactivity disorder: a pilot study. J Psychiatry Neurosci 2001;26:221-228.
- Niederhofer H. Panax ginseng may improve some symptoms of attention-deficit hyperactivity disorder. J Diet Suppl 2009;6:22-27. https://doi.org/10.1080/19390210802687221
- Russell VA. Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr Protoc Neurosci 2011;Chapter 9:Unit9.35.
- Niederhofer H. First preliminary results of an observation of Panax ginseng treatment in patients with autistic disorder. J Diet Suppl 2009;6:342-346. https://doi.org/10.3109/19390210903280231
- Wang J, Flaisher-Grinberg S, Li S, Liu H, Sun L, Zhou Y, Einat H. Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice. J Ethnopharmacol 2010;132:65-69. https://doi.org/10.1016/j.jep.2010.07.042





