Extremely high abundances of Prasiola crispa-associated micrometazoans in East Antarctica


Опубликована Июнь 20, 2022
Последнее обновление статьи Июль 26, 2023
Abstract
To elucidate poorly known aspects of the microscopic metazoan distribution in ice-free parts of the Antarctic, we examined samples of the multicellular terrestrial alga Prasiola crispa, collected over the last decade in different parts of continental East Antarctica and Haswell Island. We found that the micrometazoans inhabiting the algae consist of remarkably abundant bdelloid rotifers (subclass Bdelloidea), followed by tardigrades. We did not find nematodes. The rotifer assemblages were characterized by low diversity (only six species). Nevertheless, rotifer densities were extremely high: mean densities ranged from 75 to 3030 individuals per 100 mg of the dry sample weight and the maximum value numbered in excess of 8000 per 100 mg of the dry sample weight. These data show that terrestrial algae, along with mosses, are a very attractive habitat for rotifers and tardigrades in the Antarctic. The statistical analysis showed a lack of correlations between rotifer and tardigrade densities and nutrients (N, C, P, K and Na). Our findings are consistent with the patchy distribution of terrestrial micrometazoans in the Antarctic that has previously been found.
Ключевые слова
Tardigrades, Bdelloids, rotifers, Antarctic oases, nematodes, algae
Introduction
Terrestrial life in the Antarctic is concentrated in the few ice-free areas; is well adapted to extremely low temperatures, strong winds, freeze–thaw cycling and nutrient deficiency (Convey 1996; Rogers et al. 2012); is highly endemic (Pugh & Convey 2008; Convey et al. 2020) and has a long evolutionary history (Convey & Stevens 2007; Convey et al. 2008; Fraser et al. 2012; Convey et al. 2020). Microscopic metazoans, which include rotifers, nematodes and tardigrades, are important components of terrestrial species assemblages and inhabit both soils, the so-called ‘chalikosystems’ proposed by Janetschek (1963, 1967), and microbiotopes, forming in moss cushions, lichen and algal thalli (the so-called ‘bryosystems’). Simple intraspecific interactions and low taxonomic diversity allow for the consideration of terrestrial micrometazoan assemblages as models for ecology studies (Adams et al. 2006; Wall et al. 2006; Heatwole & Miller 2019). This is especially important in the context of climate change and anthropogenic impact, both of which are dramatically affecting the components of Antarctic biota (Convey 2010; Chown et al. 2012; Convey & Peck 2019).
The Antarctic terrestrial biota is commonly considered to be relatively poorly known (Convey 2010; Convey et al. 2014). Nevertheless, a number of genetic studies of micrometazoans have enhanced our knowledge of the diversity of particular groups (Velasco-Castrillón, Page et al. 2014; Velasco-Castrillón & Stevens 2014; Velasco-Castrillón et al. 2015) and have revised earlier published data (Iakovenko et al. 2015; Velasco-Castrillón et al. 2018). Other publications pertaining to micrometazoan diversity have a more global basis and describe general patterns (e.g., Fontaneto et al. 2015), with some offering a review of the literature (e.g., Adams et al. 2006; Velasco-Castrillón, Gibson et al. 2014) or a data synopsis (Garlasché et al. 2020).
The ecological patterns of Antarctic micrometazoans are characterized by great variability and inconsistency. Serial surveys conducted in both the chaliko- and bryosystems of Dronning Maud Land found no (Sohlenius et al. 1995; Sohlenius et al. 2004) or very little (Sohlenius et al. 1996; Sohlenius & Boström 2005) correlation between the presence of large micrometazoan taxa and biotic and abiotic factors. This has led to the conclusion that the micrometazoan distribution in Antarctica is largely a result of colonization processes and historical factors, apart from the environmental conditions (Sohlenius & Boström 2008). However, in a more recent study, micrometazoans in the Larsemann Hills (Heatwole & Miller 2019) were found to have habitat preferences, and some groups of micro-invertebrates were strongly associated with one another in terms of abundance and diversity. Studies of the microbial mats of maritime Antarctica (Velázquez et al. 2017; Almela et al. 2019) and the soils of the McMurdo Dry Valleys (Shaw et al. 2018) have shed light on the general outlines of the trophic interactions and nutrient fluxes among the main micrometazoan taxa. Moreover, another recent study has found, in contrast to earlier studies, that the nematode occurrence in soils in this region is determined by biotic interactions (Caruso et al. 2019) with geochemistry and altitude (Zawierucha et al. 2019) and by moisture (Andriuzzi et al. 2018). Soil chemistry and moisture were correlated with the diversity and distribution of micrometazoans at Edmonson Point, where a predominance of rotifers and their species-specific habitat preferences were reported (Smykla et al. 2018). In general, the distribution of soil microfauna in the Antarctic is patchy and appears to be determined by soil chemistry rather than geography (Velasco-Castrillón, Schultz et al. 2014). The sometimes contradictory findings across different habitats and regions underscore the necessity of further investigations.
In our study, reported herein, we focused on terrestrial algae, which are among the substrates that have not been well studied in connection with micrometazoans. We examined samples of the commonly found green macroalga, Prasiola crispa (Lightfoot) Kützing 1843, collected during the 2011–19 summer seasons at different sites in continental East Antarctica and on Haswell Island. We observed extremely abundant micrometazoans (rotifers first and then tardigrades). We consider several issues here: (1) the structure of the micrometazoan community in the algae; (2) how habitat characteristics appear to be linked with the high abundance of inhabiting animals, especially the rotifers; (3) a comparison of the observed densities and diversity with those of other Antarctic regions and types of habitats.
Materials and methods
Characteristics of study material
The study material included lumps of the terrestrial algae, Prasiola crispa, along with the underlying ground substrate, from different parts of East Antarctica (Fig. 1). We collected samples at three sites in the Thala Hills, in Enderby Land, near the Belarusian station Vechernyaya Mount. We also collected samples at two sites in the Larsemann Hills, near the Russian station Progress. Algae were collected manually during the 2012/13, 2016/17 and 2018/19 summers (Table 1). Algal growth in a variety of settings was sampled, including ground depressions, crevices among rocks and, in the case of sites in the Larsemann Hills, pond-like hollows (Fig. 2). Between seven and 24 samples, each about 2.0 cm3 in size, were collected at sites in different parts of the algal growth. We also examined older material, which had been collected at one site on Haswell Island and one site in the vicinity of the Druzhnaya Russian field station by Sandefjord Bay during the 2010/11 season. Old photographs and oral descriptions revealed that these sites strongly resembled those from which samples had been collected between 2012 and 2019. Seven samples of the 2010/11 specimens from near Druzhaya and 24 samples from the 2010/11 specimens collected on Haswell Island were examined. These older samples were similar in size to the samples collected between 2012 and 2019. Algae were stored frozen (collected in 2016/17 and 2018/19) or dried (collected in 2012/13 and the older specimens, collected in 2010/11), without being chemically preserved. All materials have been deposited in the collections of the Scientific and Practical Centre for Bioresources (Belarus).
Table 1 Information about sampling sites. | |||||||
No. | Site | Region | Coordinates (decimal) | Altitude (m a.s.l.) | No. of samples | Sampling date | Collected by |
1 | Haswell | Haswell Island | No exact data | No exact data | 24 | Jan 2011 | Y. Hihiniak |
2 | Druzhnaya | Sanderfjord Bay | No exact data | No exact data | 7 | Jan 2011 | Y. Hihiniak |
3 | Vechernyaya1 | Thala Hills | S 67.65972 E 046.14619 | 147 | 8 | Jan 2012 | V. Miamin |
4 | Vechernyaya2 | Thala Hills | S 67.66117 E 046.14148 | 109 | 7 | Dec 2018 | D. Lukashanets |
5 | Vechernyaya3 | Thala Hills | S 67.65577 E 046.15339 | 42 | 12 | Jan 2019 | D. Lukashanets |
6 | Progress1 | Larsemann Hills | S 69.37593 E 076.39262 | 7 | 24 | Feb 2017 | Y. Hihiniak |
7 | Progress2 | Larsemann Hills | S 69.38041 E 076.39259 | 12 | 20 | Feb 2017 | Y. Hihiniak |


Sample processing
Before analysis, all stones, large sand particles, penguin feathers and other inclusions were removed from the samples. The concentrations of the main elements—the different nutrients—were measured in the samples for each site (for details, see the Supplementary material).
After hydrating the samples, we observed numerous individuals of the phyla Rotifera and Tardigrada. To count all the individuals, we used the method suggested by Peters et al. (1993) for the extraction of rotifers from mosses, which constitutes multiple washings (up to 10) and counting the number of animals in each washing. For each washing, we shook the samples vigorously in a 50-ml vial with 20 ml of distilled water. This procedure allowed for the almost complete disintegration of the sample, making all metazoans visible (Fig. 3).

Abundance values, which had been estimated by the simple counting of all animals in the Petri dish for each washing of the sample of known weight, were then recalculated as densities in ind·0.1 gdw-1. Although recalculating to the area each sample could cover is more conventional for quantitative estimates of microscopic animals, we preferred the ‘per weight’ approach, largely because of the complexity of the Prasiola crispa thallus. Even differences of a few millimetres in algal thickness can result in a significant increase in the animal number, making it very complicated to standardize the sample by its square.
One hundred revived rotifer individuals were chosen randomly and were then transferred to slides and examined using light microscopy (Nikon and Micros). These living, active animals were examined with microphotography (NIS Elements, Microscopy Vision software). We used the most convenient keys (Donner 1965; Kutikova 2005) and articles that contain information regarding Antarctic bdelloid identification (Iakovenko et al. 2015). Additionally, we used the morphometry analysis suggested by Iakovenko et al. (2013) and the DNA sequencing of the COXI mitochondrial gene (Supplementary Table S3). Tardigrades, initially not the focus of the research (many of them were in an unidentifiable state), were studied only superficially and were identified to higher taxa using the appropriate keys (Ramazzotti & Maucci 1983; Pilato & Binda 2010; Bingemer & Hohberg 2017).
Data analysis
Spearman correlation tests of rotifer and tardigrade density values against nutrient content and rotifer density values against tardigrade density values were performed using R statistics (R-Studio integrated development environment) and PAST software for scientific data analysis.
Results
Sample characteristics
All samples comprised algal thalli with particles of the hardly visible ground and extraneous organic particles, which remained after primary processing. The samples varied significantly in terms of the Na, P and K contents, whereas N and C concentrations did not differ much (Table 2).
Table 2 Nutrient concentrations at sampling sites. | ||||||
No. | Site | Na (g/kg) | P (g/kg) | K (g/kg) | N (%) | C (%) |
1 | Haswell | 59.47 | 8.56 | 86.81 | 3.32 | 31.35 |
2 | Druzhnaya | 155.95 | 14.736 | 105.61 | 3.35 | n/d |
3 | Vechernyaya1 | 34.32 | 9.78 | 194.83 | 3.68 | 39.98 |
4 | Vechernyaya2 | 56.41 | 17,25 | 200.53 | 2.08 | 25.81 |
5 | Vechernyaya3 | 60.64 | 10.13 | 118.28 | 2.80 | 28.23 |
6 | Progress1 | 100.55 | 7.11 | 202.29 | 3.49 | 32.44 |
7 | Progress2 | 31.76 | 2.338 | 133.28 | 3.91 | 39.36 |
Taxonomic diversity
The animals in the algal samples included bdelloid rotifers (Rotifera: Bdelloidea) and tardigrades (Tardigrada). Nematodes, the third main group of the micrometazoan community in the Antarctic, were absent.
Species identification of bdelloids was possible only in the case of frozen samples (from three sites: Progress1, Progress2 and Vechernyaya3) that recovered from cryptobiosis almost completely. Most of the individuals in the dried samples retained their unidentifiable tun shape after hydration.
All notes relating to the identification of the rotifer species are presented in the Supplementary material.
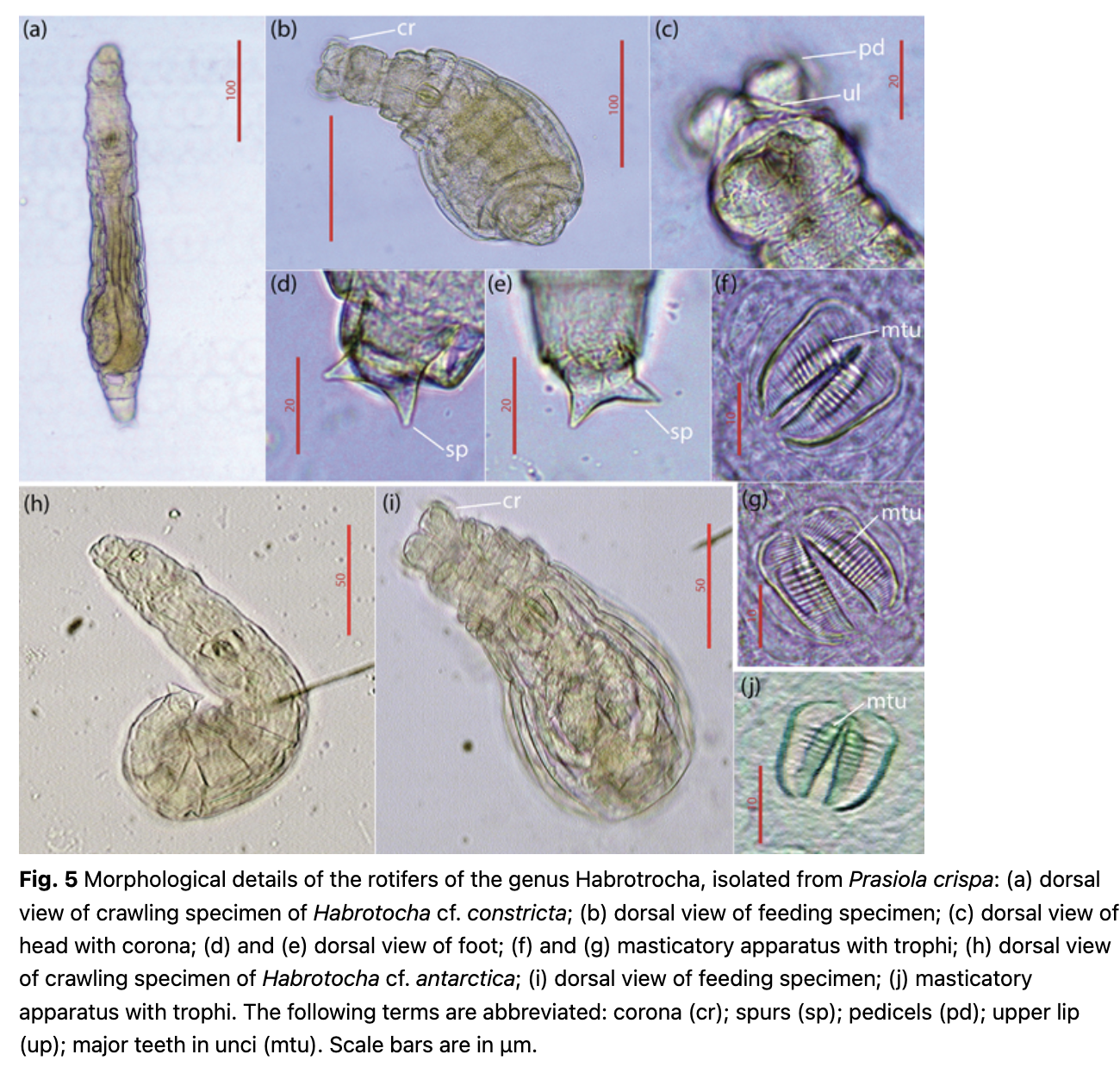
Morphological examination of the bdelloids revealed six morphotypes. Four can be identified as Antarctic species. Adineta grandis (Murray 1910) and Philodina gregaria (Murray 1910) were recorded at Progress1; Adineta emsliei (Iakovenko 2015) and Habrotrocha antarctica (Iakovenko 2015) were recorded at Progress2. It is noteworthy that the morphometric analysis showed differences between the morphotypes found and the “type varieties” discussed by Iakovenko (2015; Supplementary Table S4). On the basis of morphology, the other two species found in samples from the Vechernyaya3 site can be considered as belonging to the “Habrotrocha constricta” (Dujardin 1841) species group and to the “Adineta vaga” (Davis 1873) species group, both of which are cosmopolitan taxa; the precise status of which is under debate (Figs. 4–6).


DNA sequencing was carried out on the specimens identified as A. grandis and Ph. gregaria from Progress1 (Supplementary Table S5). The analysis using BLAST fully supported the morphological identification. DNA samples, which were extracted from individuals with the Ph. gregaria morphotype (GenBank identification OK325599 and MT584979), can be attributed to the species with 98–100% certainty, according to the BLAST search. DNA samples from the specimen identified, on the basis of morphology, as A. grandis (GenBank ID OK325600) referred to the said species with more than 99% similarity (Supplementary Fig. S1).
As identification without the analysis of nucleotide sequences is arguable, we indicated the other four species as “cf.” Such ambiguity is especially suitable in relation to those species which we initially linked with A. vaga and H. constricta.
All tardigrades were Eutardigrada members. The genera Macrobiotus, Mesobiotus and Diphascon have been identified in the samples collected over the last two seasons.
Abundance
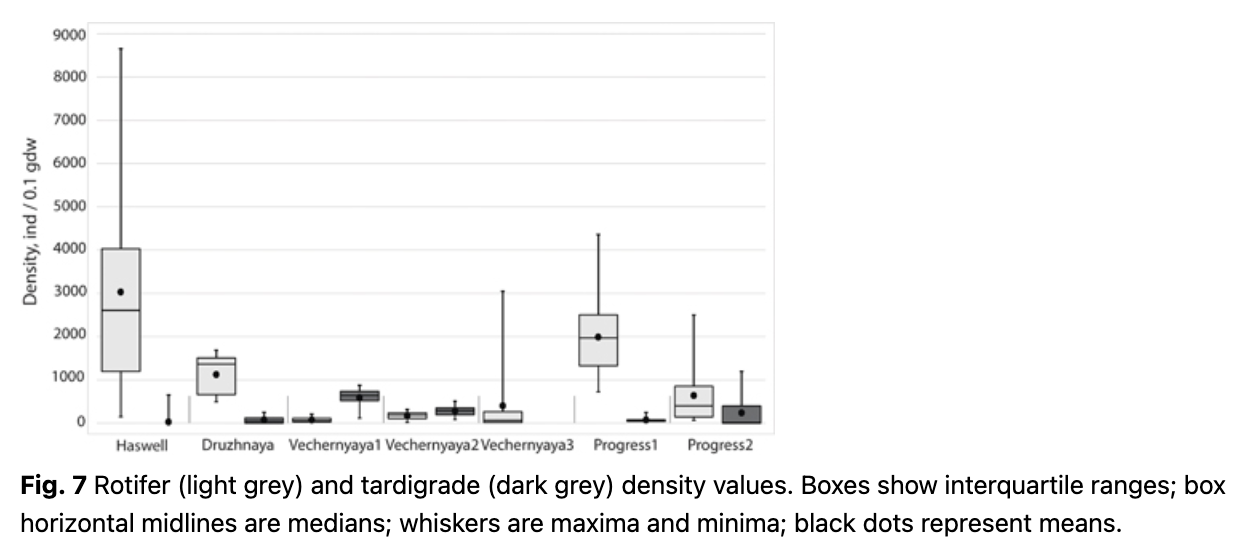
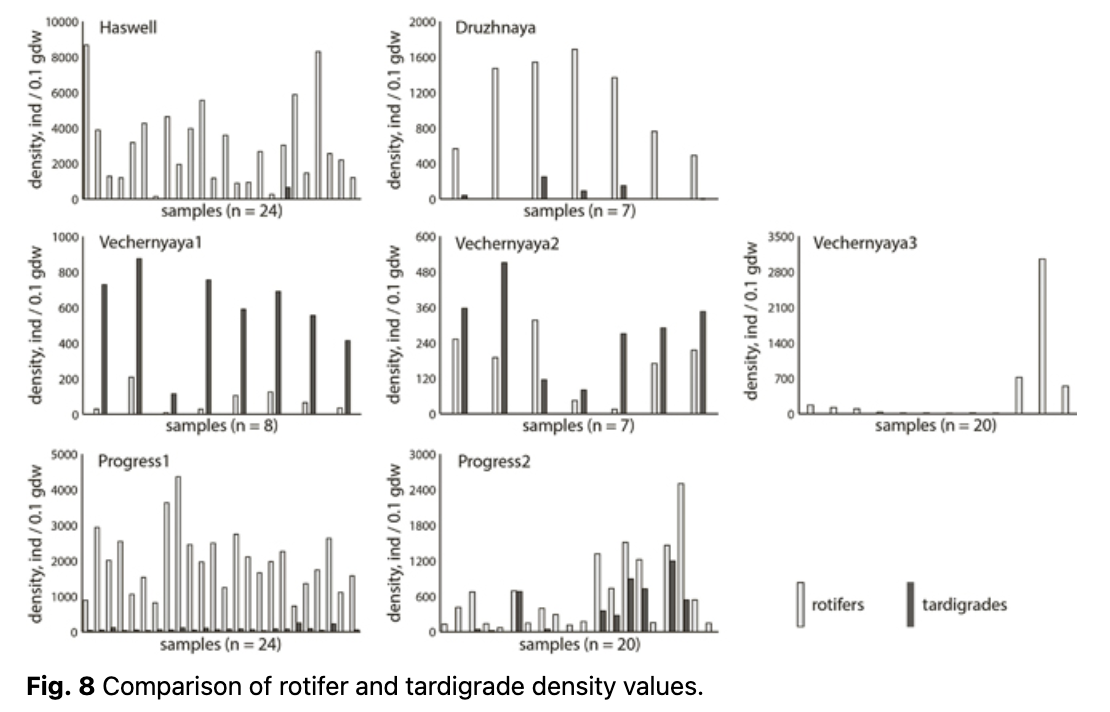
High abundances of micrometazoans were found at all the studied sites (Fig. 7). Assemblages in five of the seven sites consisted mainly of bdelloid rotifers (72.8–100% of individuals), while at Vechernyaya1 and Vechernyaya2 tardigrades predominated (88.7 and 62.0% of individuals, respectively). The sites with the highest densities (with mean and median values over 1000 ind·0.1 gdw-1) were the rotifer-dominated sites—Druzhnaya, Haswell and Progress1—where rotifer mean densities ranged from 1125 to 3030 ind·0.1 gdw-1, and the maximal value was about 8650 ind·0.1 gdw-1. The tardigrade densities at the two tardigrade-dominated sites—Vechernyaya1 and Vechernyaya2—were lower by an order of magnitude and yielded 590 and 281 ind·0.1 gdw-1, which was comparable to that of the rotifers at Vechernyaya3 and Progress2: 400 and 639 ind·0.1 gdw-1 (mean values), respectively. At Vechernyaya3, the range of values among samples at a particular site was notable: the maximum exceeded 3000 ind·0.1 gdw-1.
Haswell and Progress2 were almost barren of tardigrades, whilst no tardigrades were detected at all at Vechernyaya3. In contrast, rotifers were found in every sample at each site. Rotifer and tardigrade densities did not correlate across the samples within each site, except for Progress2, where they demonstrated a high Spearman’s rank correlation coefficient between the two groups, with a significant p value level (Fig. 8; Table 3).
Table 3 Correlations between rotifer and tardigrade densities. The significant correlation is in boldface. | ||||
No. | Site | Correlation indices |
| |
Ra | pb |
| ||
1 | Haswell | 0.18 | 0.39 |
|
2 | Druzhnaya | 0.65 | 0.12 |
|
3 | Vechernyaya1 | 0.41 | 0.33 |
|
4 | Vechernyaya2 | 0.29 | 0.56 |
|
5 | Vechernyaya3 | - | - |
|
6 | Progress1 | 0.16 | 0.46 |
|
7 | Progress2 | 0.82 | 0.000012 |
|
aSpearman’s rank correlation coefficient. bSignificance. |
| |||
The correlation analysis of the nutrient content in the samples and the abundance values of the rotifers and the tardigrades did not indicate possible links in any comparison case (Table 4).
Table 4 Correlations between micrometazoan densities and nutrient concentrations across all sites. | |||||||||||
Taxa | Na | P | K | N | C |
| |||||
Ra | pb | Ra | pb | Ra | pb | Ra | pb | Ra | pb |
| |
Rotifers | 0.50 | 0.27 | −0.42 | 0.35 | −0.39 | 0.39 | 0.04 | 0.96 | −0.09 | 0.92 |
|
Tardigrades | −0.57 | 0.20 | 0.14 | 0.78 | 0.46 | 0.30 | 0.35 | 0.44 | 0.43 | 0.42 |
|
All | 0.28 | 0.56 | −0.50 | 0.27 | −0.25 | 0.59 | 0.32 | 0.49 | 0.37 | 0.49 |
|
aSpearman’s rank correlation coefficient. bSignificance. |
| ||||||||||
Discussion
The first description of the very high rotifer abundances in the terrestrial ecosystems of Antarctica is to be found in Murray’s pioneer zoological surveys (1910a, b). He observed (1910b: 18): “I have never anywhere seen Bdelloid rotifers so plentiful as are the two dominant species at Cape Royds (Philodina gregaria and Adineta grandis). Among the higher Invertebrata, the Rotifera are easily first in numbers, both of individuals and species.” This work lacks detailed information regarding the habitat that was so abundantly populated by bdelloids; however, Murray was probably referring to multicellular algae (according to his description, very similar to Prasiola crispa) and definitely not mosses.
More recent studies showed that P. crispa is inhabited by rotifers (Suren 1990; Everitt 1981) and tardigrades (Dougherty & Harris 1963; Miller et al. 1988; Miller, Heatwole et al. 1994; Miller & Heatwole 1996; Sohlenius et al. 2004; Heatwole & Miller 2019). Other small animals are found in P. crispa (Sinclair et al. 2006; Dalto et al. 2010; De Mendonça et al. 2012; Gantz et al. 2018), including Belgica antarctica, the Antarctic endemic insect (Sugg et al. 1983). It is likely that terrestrial algae provide favourable microhabitats for invertebrates, and some results indicate that arthropods prefer algae to mosses because of the feeding conditions (Bokhorst et al. 2007). In Svalbard, in the Arctic, the P. crispa coverage near depositions of seabird guano is one of the most important environmental factors that positively affect the diversity and the abundance of springtails (Zmudczyńska et al. 2012). However, since Murray’s work, no one has confirmed the huge abundances of invertebrate animals in algae, with the exception of one study (Schulte et al. 2008), which documented a high density of Antarctic collembola eggs, presumably as a consequence of global warming.
Our finding of thousands of individual bdelloid rotifers in a very limited algal volume illustrates the poorly described phenomenon. The density values per dry weight unit that we found are higher by orders of magnitude than those reported in the literature for algae, lichens, soil and—the most interesting—mosses (Table 5). Bryophytes are sometimes claimed to be the preferred habitat for Antarctic micrometazoans (e.g., Porazinska et al. 2004; Sohlenius et al. 2004; Smykla et al. 2010). In the Larsemann Hills, it has been shown that all micrometazoan taxa occur more frequently in mosses, and they avoid soil (Heatwole & Miller 2019). Unfortunately, our findings cannot be compared with micrometazoan density results that were assessed using other types of standardization, for example, individuals per area or individuals in a core. However, roughly extrapolating between the sample volumes analysed in other studies (e.g., the 3.0 × 3.5 cm cores used by Jennings [1976]) and the much smaller sample volumes analysed in the present study suggests values that are similar or at least of the same order of magnitude. Unfortunately, there were no quantitative data in Murray’s work (1910a, b), which was the first to report high rotifer abundances in Antarctic algae.
Table 5 Micrometazoan density values (ind·0.1 gdw-1), comparing data obtained in the present study to the literature. Some values taken from the literature were recalculated to ind·0.1 gdw-1 from individuals per other weight units (g, 100 g, etc.). | ||||||||||||||||||
Taxa | HIa | LHb | THc | VHd | DMe | DMf | DMg | DMh | DMi | RIj | ELk | VLl | VLm | DVn | DVo | DVp | DGq |
|
Algae | Other bryosystem substrata (e.g., moss) | Soil |
| |||||||||||||||
Rotifers |
| |||||||||||||||||
Mean | 3030 | 1251 | 216 | ca. 16s | 87 | 1–15r | 5 | 5 | 2–51r | 0–<1r | ndt | 1 | 3 | ndt | 0–<1r | <1 | – |
|
Max | 8656 | 4360 | 3049 | ca. 40s | 272 | ca. 45s | 75 | 75 | 425 | <1 | ndt | 8 | 34 | ndt | <1 | n/d | – |
|
Tardigrades |
| |||||||||||||||||
Mean | 29 | 129 | 290 | ca. 39s | 2 | <1–2r | 4 | 1 | <1–4r | 0–<1r | <1 | <1 | <1 | ndt | 0–<1r | <1 | – |
|
Max | 647 | 1195 | 875 | ca. 49s | 3 | ca. 20 | 58 | 36 | 240 | <1 | <1 | 2 | 3 | ndt | <1 | ndt | – |
|
Nematodes |
| |||||||||||||||||
Mean | – | – | – | ndt | 123 | <1–3r | 3 | 3 | <1–50r | 0–<1r | ndt | <1 | <1 | 0–<1r | <1 | <1 | <1 |
|
Max | – | – | – | ndt | 506 | ca. 20s | 21 | 59 | 506 | <1 | ndt | 2 | 3 | <1 | <1 | ndt | <1 |
|
Totals |
| |||||||||||||||||
Mean | 3059 | 1380 | 506 | ndt | ndt | 1–20r | ndt | ndt | 3–102r | 0–<1r | ndt | ndt | 4 | ndt | <1 | <1 | <1 |
|
Max | 8656 | 4470 | 3049 | ndt | ndt | ndt | ndt | ndt | 781 | <1 | ndt | ndt | 35 | ndt | <1 | ndt | <1 |
|
aHaswell Island; present study. bLarsemann Hills & Sanderfjord Bay; present study. cThala Hills; present study. dVestfold Hills; Everitt 1981. eDronning Maud Land; Sohlenius et al. 2004. fDronning Maud Land; Sohlenius et al. 1995. gDronning Maud Land; Sohlenius et al. 1996. hDronning Maud Land; Sohlenius & Boström 2005. iDronning Maud Land; Sohlenius & Boström 2008. jRoss Island; Porazińska et al. 2002a. kEllsworth Land Convey & McInnes 2005. lVictoria Land; Smykla et al. 2010. mVictoria Land; Smykla et al. 2018. nAntarctic Dry Valleys; Porazińska et al. 2002b. oAntarctic Dry Valleys; Andriuzzi et al. 2018. pAntarctic Dry Valleys; Shaw et al. 2018. qDarwin Glacier; Zawierucha et al. 2019. rRange reflects the several types of estimated habitats/areas. sApproximate values (taken from figures). tNo data. |
| |||||||||||||||||
Four of the six species of rotifers found in the present study are Antarctic endemics. Philodina gregaria and Adineta grandis, found on the continent during the earliest zoological surveys (Murray 1910a), have large sizes (up to 700 µm), are viviparous and are conspicuous because of their pigmentation: A. grandis is bright red and Ph. gregaria is dark red. The correct A. grandis identification was possible only by applying mtDNA sequencing, as A. fontanetoi (Iakovenko 2015) is a morphological twin of A. grandis and these can only be distinguished from one another genetically. Adineta emsliei and Habrotocha antarctica were recently described as a result of an in-depth study of Antarctic bdelloids (Iakovenko et al. 2015). Despite the fact that both Habrotocha constricta and Adineta vaga had been formally noted in the Antarctic (Segers 2007), identifying them on the basis of morphology alone is problematic. Application of DNA taxonomy methods, along with analysis of the trophi microstructure, has demonstrated that Antarctic representatives are distinct from their relatives elsewhere in the world (Iakovenko et al. 2015). The situation in relation to A. vaga is particularly complicated as this name has been assigned to a species that should either be re-described as a species group or considered unidentifiable (Örstan 2020). The difference between the supposed member of the A. vaga complex in the present study and A. emslei—the Antarctic variant of A. vaga (Iakovenko et al. 2015)—underscores the need for modern molecular approaches.
As tardigrades and rotifers in the Antarctic often co-exist with nematodes (e.g., Sohlenius et al. 2004), the utter absence of the latter is a very unexpected result of the study. These organisms—the most abundant and diverse group of metazoans on Earth (Poinar 2015; van den Hoogen et al. 2019)—are widespread in the Antarctic (e.g., Velasco-Castrillón, Gibson et al. 2014; Velasco-Castrillón & Stevens 2014), including hostile Antarctic deserts, where nematodes can be the only metazoan taxon present (Zawierucha et al. 2021). Nematodes can dominate in algae (e.g., Sohlenius et al. 2004) and in bacterial mats, playing a key role as top consumers (Almela et al. 2019). Other than the present study, micrometazoan communities without nematodes are exemplified only in cryoconite holes, which are unique glacier habitats formed when dark debris on the ice leads to melting. The absence of nematodes in cryoconite holes can be explained by their unsuitableness for reproduction: low temperatures and rapid shifts from oxic to anoxic conditions (Zawierucha et al. 2021). Explanations for the absence of nematodes in samples of terrestrial alga Prasiola crispa, as we found, require further investigation.
The extremely high abundance of rotifers and tardigrades shown in the present study can be considered as a particular case of the patchy spatial distribution of Antarctic micrometazoans (Miller, Miller et al. 1994; Adams et al. 2006). Microsites with high abundance values, such as samples in the present study, may be interspersed with those where micrometazoan abundance is low to moderate or micrometazoans may be absent altogether, even within a homogeneous biotope. As the data set in the present study is limited, we need further research in this direction to elucidate this pattern. However, some preliminary ideas regarding to micrometazoan extreme abundances in Prasiola crispa can be outlined.
The specific conditions of terrestrial algae may be facilitating an increase in micrometazoans. The chemical composition of the substrate seems to be an important factor affecting the distribution of animals dwelling there. The organic matter content and C/N ratio do not influence the micrometazoan community across several types of habitats (Sohlenius et al. 1996). In Antarctic soils, strong nutrient contamination by bird faeces (high N content) inhibits tardigrades (Sohlenius et al. 2004; Smykla et al. 2012); likewise, nematodes have a limited presence in ornithogenic soils in active penguin rookeries (Porazińska et al. 2002a). However, rotifers appear to be associated with areas of higher organic content (Sinclair & Sjursen 2001; Sohlenius et al. 2004). Bokhorst et al. (2019) demonstrate increases in the abundance and diversity of moss- and lichen-dwelling tardigrades and micro-arthropods with nitrogen input from Antarctic marine vertebrates. In the Arctic, the picture seems to be clearer: invertebrate communities are more abundant in habitats enriched by seabird guano (Zmudczyńska et al. 2012; Zmudczyńska-Skarbek et al. 2015; Zawierucha et al. 2016; Zmudczyńska-Skarbek et al. 2017). However, all these studies neglected rotifers. In our study, most of sites presented the places Adélie penguin (Pygoscelis adeliae) moulting. We expected to find more micrometazoans at the sites where there were more expressed signs of ‘ornithogeneity,’ but this factor was not estimated quantitatively. Nonetheless, we demonstrated a lack of correlations between nutrient content and animal abundance (Table 4). It may be that the microscopic animal communities are mostly determined by completely different chemistry-independent factors, while the observed variability of element concentration in the samples, caused by the presence of inconspicuous mineral particles (sand grains), does not influence the abundance of animals that live in algae.
The explanation may possibly lie in the algal surface, which is structurally complicated, offering many microhabitats for tiny animals. Another important aspect that may have a positive influence on abundance is the water content around the algae. The existence of Antarctic invertebrates is limited by liquid water, which is the main driver, critically influencing their dispersal (Block et al. 2009; Convey et al. 2014). All samples were collected during the summer season (December–February), when bryosystems experienced their highest saturation with thawing water. We suggest that the water film that forms on and around the algal thalli may serve as a spacious environment for micrometazoans. This may help account for the predominance of bdelloid rotifers over tardigrades. These animals, unlike tardigrades, filter sediment and can easily feed on bacteria and other microscopic food in a liquid environment. Moreover, many rotifers are semi-attached animals that alternate between swimming while feeding and crawling or sitting on a firm surface. Hydration, together with the availability of food, creates a superior environment for them, causing the population to increase rapidly in terms of number and biomass. The fact that rotifers and tardigrades require different conditions is illustrated by our results: almost no correlations between these two were found. However, others have found correlations between rotifer and tardigrade abundances (Sohlenius & Boström 2005, 2008).
Another consideration is the bryosystem characteristics on a broader scale. The sites Progress1 and Progress2 have voluminous conglomerations of algae in deep hollows, as opposed to the thin algae layers on ground or rock surfaces that we commonly encountered during our collecting. Deep hollows can foster the continuous accumulation of animals, as new algal layers grow. The cumulative effect of this process over years, allowing for periods of abundance (summer) interspersed with dormancy periods (winter), has resulted in millions of rotifers.
Conclusion
We observed remarkably high abundances of bdelloid rotifers, followed by tardigrades, in algal samples collected at different East Antarctic oases. These values far exceeded those available in the literature for algae and other bryosystems components and soil. Counter to our expectations, we found no nematodes. We believe that the irregular distribution of micrometazoans in our samples does not relate to nutrient content but, instead, is likely to be associated with algal surface characteristics, moisture content and/or the history of the habitat formation.
Acknowledgements
The authors would like to thank the members of the Belarusian Antarctic Expeditions who assisted in the sample collection and the delivery of the material from the Antarctic, especially Alexey A. Gaidashov, the chief of expeditions, for his essential support in field surveys. The authors appreciate the contribution of Konstantin Homel (DNA isolation and amplification), Maria Sasinovich (DNA sequencing), Yelena Kasyanik (N and C estimation in samples) and Sergey Zuy (Na, P and K estimation in samples). The authors specially thank Professor Galina A. Galkouskaya, for raising interest with regard to the study of Bdelloidea rotifers. The authors also thank Dr Nataliia S. Iakovenko and an anonymous reviewer for comments that improved the manuscript.
References
- Adams B.J., Bardgett R.D., Ayres E., Wall D.H., Aislabie J., Bamforth S., Bargagli R., Cary C., Cavacini P., Connell L., Convey P., Fell J.W., Frati F., Hogg I.D., Newsham K.K., O’Donnell A., Russell N., Seppelt R.D. & Stevens M.I. 2006. Diversity and distribution of Victoria Land biota. Soil Biology and Biochemistry 38, Spec. Issue S1, 3003–3018, doi: 10.1016/j.soilbio.2006.04.030.
- Almela P., Velázquez D., Rico E., Justel A. & Quesada A. 2019. Carbon pathways through the food web of a microbial mat from Byers Peninsula, Antarctica. Frontiers in Microbiology 10, article no. 628, doi: 10.3389/fmicb.2019.00628.
- Andriuzzi W.F., Adams B.J., Barrett J.E., Virginia R.A. & Wall D.H. 2018. Observed trends of soil fauna in the Antarctic Dry Valleys: early signs of shifts predicted under climate change. Ecology 99, 312–321, doi: 10.1002/ecy.2090.
- Bingemer J. & Hohberg K. 2017. An illustrated identification key to the Eutardigrade species (Tardigrada, Eutardigrada) presently known from European soils. Soil Organisms 89, 127–149.
- Block W., Lewis Smith R.I. & Kennedy A.D. 2009. Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biological Reviews 84, 449–484, doi: 10.1111/j.1469-185X.2009.00084.x.
- Bokhorst S., Convey P. & Aerts R. 2019. Nitrogen inputs by marine vertebrates drive abundance and richness in Antarctic terrestrial ecosystems. Current Biology 29, 1721–1727, doi: 10.1016/j.cub.2019.04.038.
- Bokhorst S., Ronfort C., Huiskes A., Convey P. & Aerts R. 2007. Food choice of Antarctic soil arthropods clarified by stable isotope signatures. Polar Biology 30, 983–990, doi: 10.1007/s00300-007-0256-4.
- Caruso T., Hogg I.D., Nielsen U.N., Bottos E.M., Lee C.K., Hopkins D.W., Cary S.C., Barrett J.E., Green T.G.A., Storey B.C., All D.H. & Adams B.J. 2019. Nematodes in a polar desert reveal the relative role of biotic interactions in the coexistence of soil animals. Communications Biology 2, article no. 63, doi: 10.1038/s42003-018-0260-y.
- Chown S.L., Lee J.E., Hughes K.A., Barnes J., Barrett P.J., Bergstrom D.M., Convey P., Cowan D.A., Crosbie K., Dyer G., Frenot Y., Grant S.M., Herr D., Kennicutt M.C. II, Lamers M., Murray A., Possingham H.P., Reid K., Riddle M.J., Ryan P.G., Sanson L., Shaw J.D., Sparrow M.D., Summerhayes C., Terauds A. & Wall D.H. 2012. Challenges to the future conservation of the Antarctic. Science 337, 158–159, doi: 10.1126/science.1222821.
- Convey P. 1996. Overwintering strategies of terrestrial invertebrates in Antarctica—the significance of flexibility in extremely seasonal environments. European Journal of Entomology 93, 489–505.
- Convey P. 2010. Terrestrial biodiversity in Antarctica—recent advantages and future challenges. Polar Science 4, 135–147, doi: 10.1016/j.polar.2010.03.003.
- Convey P., Biersma E.M., Casanova-Katny A. & Maturana C.S. 2020. Refuges of Antarctic diversity. In M. Oliva & J. Ruiz-Fernández (eds.): Past Antarctica. Pp. 181–200. Burlington: Academic Press.
- Convey P., Chown S.L., Clarke A., Barnes D.K.A., Bokhorst S., Cummings V., Ducklow H.W., Frati F., Green T.G.A., Gordeon S., Griffiths H.J., Howard-Williams C., Huiskes A.H.L., Laybourn-Parry J., Lyons W.B., McMinn A., Morley S.A., Peck L.S., Guesada A., Robinson S.A., Schiaparelli S. & Wall D.H. 2014. The spatial structure of Antarctic biodiversity. Ecological Monographs 84, 203–244, doi: 10.1890/12-2216.1.
- Convey P., Gibson J., Hillenbrand C.D., Hodgson D.A., Pugh P J.A., Smellie J.L. & Stevens M.I. 2008. Antarctic terrestrial life—challenging the history of the frozen continent? Biological Reviews of the Cambridge Philosophical Society 83, 103–117, doi: 10.1111/j.1469-185X.2008.00034.x.
- Convey P. & McInnes S.J. 2005. Exceptional tardigrade-dominated ecosystems in Ellsworth Land, Antarctica. Ecology 86, 519–527, doi: 10.1890/04-0684.
- Convey P. & Peck L.S. 2019. Antarctic environmental change and biological responses. Science Advances 11, eaaz0888, doi: 10.1126/sciadv.aaz0888.
- Convey P. & Stevens M.I. 2007. Antarctic biodiversity. Science 317, 1877–1878, doi: 10.1126/science.1147261.
- Dalto A.G., de Faria G.M., de Alencar Imbassahy C.A., de Souza Campos T.M. & Yoneshigue-Valentin Y. 2010. Associated fauna of Prasiola crispa (Chlorophyta) related to penguin rookery at Arctowski (King George Island, South Shetland Islands, maritime Antarctic). INCT-APA Annual Activity Report 2010, Thematic Area 3, 188–193, doi: 10.4322/apa.2014.048.
- De Mendonça M.C., Queiroz G.C., Abrantes E.A., Dalto A.G. & Yoneshigue-Valentin Y. 2012. Collembola (Arthropoda, Hexapoda) associated to terrestrial green algae from ice-free areas in Admiralty Bay (King George Island, South Shetlands islands, Antarctica). Annual Activity Report. Science Highlights 2012, Thematic Area 2, 94–97, doi: 10.4322/apa.2014.103.
- Donner J. 1965. Ordnung Bdelloidea (Rotatoria, Rädertiere). Bestimmungsbücher zur Bodenfauna Europas. (Order Bdelloidea [Rotatoria, rotifers]. Identification books on the soil fauna of Europe.) Berlin: Akademie.
- Dougherty E.C. & Harris L.G. 1963. Antarctic Micrometazoa: fresh-water species in the McMurdo Sound Area. Science 140, 497–498, doi: 10.1126/science.140.3566.497.
- Everitt D.A. 1981. An ecological study of an Antarctic freshwater pool with particular reference to Tardigrada and Rotifera. Hydrobiologia 83, 225–237, doi: 10.1007/BF00008270.
- Fontaneto D., Iakovenko N. & De Smet W.H. 2015. Diversity gradients of rotifer species richness in Antarctica. Hydrobiologia 761, 235–248, doi: 10.1007/s10750-015-2258-5.
- Fraser C.I., Nikula R., Ruzzante D.E. & Waters J.M. 2012. Poleward bound: biological impacts of Southern Hemisphere glaciation. Trends in Ecology & Evolution 27, 462–471, doi: 10.1016/j.tree.2012.04.011.
- Gantz J.D., Spacht D.E. & Lee R.E. 2018. A preliminary survey of the terrestrial arthropods of the Rosenthal Islands, Antarctica. Polar Research 36, article no. 1500266, doi: 10.1080/17518369.2018.1500266.
- Garlasché G., Karim K., Iakovenko N., Velasco-Castrillón A., Janko K., Guidetti R., Rebecchi L., Cecchetto M., Schiaparelli S., Jersabek C., De Smet W.H. & Fontaneto D. 2020. A data set on the distribution of Rotifera in Antarctica. Biogeographia—The Journal of Integrative Biogeography 35, 17–25, doi: 10.21426/B635044786.
- Heatwole H. & Miller W.R. 2019. Structure of micrometazoan assemblages in the Larsemann Hills, Antarctica. Polar Biology 42, 1837–1848, doi: 10.1007/s00300-019-02557-6.
- Iakovenko N.S., Kašparová E., Plewka M. & Janko K. 2013. Otostephanos (Rotifera, Bdelloidea, Habrotrochidae) with the description of two new species. Systematics and Biodiversity 24, 477–494, doi: 10.1080/14772000.2013.857737.
- Iakovenko N.S., Smykla J., Convey P., Kašparová E., Kozeretska I.A., Trokhymets V., Dykyy I., Plewka M., Devetter M., Duris Z. & Janko K. 2015. Antarctic bdelloid rotifers: diversity, endemism and evolution. Hydrobiologia 761, 5–43, doi: 10.1007/s10750-015-2463-2.
- Janetschek H. 1963. On the terrestrial fauna of the Ross Sea area, Antarctica. Pacific Insects 5, 305–311.
- Janetschek H. 1967. Arthropod ecology of South Victoria Land. Entomology Antarctica 10, 205–293, doi: 10.1029/AR010p0205.
- Jennings P.G. 1976. The Tardigrada of Signy Island, South Orkney Islands, with a note on the Rotifera. British Antarctic Survey Bulletin 44, 1–25.
- Kutikova L.A. 2005. Bdelloidnye kolovratki fauny Rossii. (Bdelloid rotifers in fauna of Russia.) Moscow: The Community of Scientific Publications KMK.
- Miller J.D., Horne P., Heatwole H., Miller W.R. & Bridges L. 1988. A survey of the terrestrial Tardigrada of the Vestfold Hills, Antarctica. Hydrobiologia 165, 197–208, doi: 10.1007/BF00025588.
- Miller W.R. & Heatwole H. 1996. Tardigrades of the Australian Antarctic territories: the Windmill Islands, East Antarctica. Zoological Journal of the Linnaean Society 116, 175–184, doi: 10.1111/j.1096-3642.1996.tb02342.x.
- Miller W.R., Heatwole H., Pidgeon R.W.J. & Gardiner G.R. 1994. Tardigrades of the Australian Antarctic Territories: the Larsemann Hills, East Antarctica. Transactions of the American Microscopical Society 113, 142–160, doi: 10.2307/3226642.
- Miller W.R., Miller J.D. & Heatwole H. 1994. Tardigrades of the Australian Antarctic Territory: assessing diversity within a sample. Memoirs of the Queensland Museum 36, 137–145.
- Murray J. 1910a. Antarctic Rotifera. British Antarctic Expedition 1907–1909. Reports of the Scientific Investigations, Biology 1(3), 41–73.
- Murray J. 1910b. Microscopic life at Cape Royds. British Antarctic Expedition 1907–1909. Reports of the Scientific Investigations, Biology 1(2), 17–22.
- Örstan A. 2020. The trouble with Adineta vaga (Davis, 1873): a common rotifer that cannot be identified (Rotifera: Bdelloidea: Adinetidae). Zootaxa 4830, 597–600, doi: 10.11646/zootaxa.4830.3.8.
- Peters U., Koste W. & Westheide W. 1993. A quantitative method to extract moss-dwelling rotifers. Hydrobiologia 255/256, 339–341, doi: 10.1007/BF00025857.
- Pilato G. & Binda M.G. 2010. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2404, 1–54, doi: 10.11646/zootaxa.2404.1.1.
- Poinar G. 2015. Phylum Nemata. In J.H. Thorp & D.C. Rogers (eds.): Thorp and Covich’s freshwater invertebrates. 4th edn. Pp. 273–302. Cambridge, UK: Academic Press.
- Porazinska D.L., Fountain A.G., Nylen T.H., Tranter M., Virginia R.A. & Wall D.H. 2004. The biodiversity and biogeochemistry of cryoconite holes from McMurdo Dry Valley glaciers, Antarctica. Arctic, Antarctic, and Alpine Research 36, 84–115, doi: 10.1657/1523-0430(2004)036[0084:TBABOC]2.0.CO;2.
- Porazińska D.L., Wall D.H. & Virginia R.A. 2002a. Invertebrates in ornithogenic soils on Ross Island, Antarctica. Polar Biology 25, 569–574, doi: 10.1007/s00300-002-0386-7.
- Porazińska D.L., Wall D.H. & Virginia R.A. 2002b. Population age structure of nematodes in the Antarctic Dry Valleys: perspectives on time, space, and habitat suitability. Arctic, Antarctic, and Alpine Research 34, 159–168, doi: 10.2307/1552467.
- Pugh P.J.A. & Convey P. 2008. Surviving out in the cold: Antarctic endemic invertebrates and their refugia. Journal of Biogeography 35, 2176–2186, doi: 10.1111/j.1365-2699.2008.01953.x.
- Ramazzotti G. & Maucci W. 1983. Phylum Tardigrada. Memorie dell’Istituto Italiano di Idrobiologia 41, 1–1012.
- Rogers A.D., Johnston N.M., Murphy E.J. & Clarke A. 2012. Antarctic ecosystems: an extreme environment in a changing world. Oxford: Wiley-Blackwell.
- Schulte G., Elnitsky M., Benoit J., Denlinger D. & Lee R. 2008. Extremely large aggregations of collembolan eggs on Humble Island, Antarctica: a response to early seasonal warming? Polar Biology 31, 889–892, doi: 10.1007/s00300-008-0445-9.
- Segers H. 2007. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564, 1–104, doi: 10.11646/zootaxa.1564.1.1.
- Shaw E.A., Adams B.J., Barrett J.E., Lyons W.B., Virginia R.A. & Wall D.H. 2018. Stable C and N isotope ratios reveal soil food web structure and identify the nematode Eudorylaimus antarcticus as an omnivore-predator in Taylor Valley, Antarctica. Polar Biology 41, 1013–1018, doi: 10.1007/s00300-017-2243-8.
- Sinclair B.J., Scott M.B., Jaco Klok C., Terblanche J.S., Marshall D.J., Reyers B. & Chown S.L. 2006. Determinants of terrestrial arthropod community composition at Cape Hallett, Antarctica. Antarctic Science 18, 303–312, doi: 10.1017/S0954102006000356.
- Sinclair B.J. & Sjursen H. 2001. Terrestrial invertebrate abundance across a habitat transect in Keble Valley, Ross Island, Antarctica. Pedobiologia 45, 134–145, doi: 10.1078/0031-4056-00075.
- Smykla J., Iakovenko N., Devetter M. & Kaczmarek L. 2012. Diversity and distribution of tardigrades in soils of Edmonson Point (northern Victoria Land, continental Antarctica). Czech Polar Reports 2, 61–70, doi: 10.5817/CPR2012-2-6.
- Smykla J., Porazinska D., Iakovenko N., Devetter M., Drewnik M., Hii Y. & Emslie S. 2018. Geochemical and biotic factors influencing the diversity and distribution of soil microfauna across ice-free coastal habitats in Victoria Land, Antarctica. Soil Biology and Biochemistry 116, 265–276, doi: 10.1016/j.soilbio.2017.10.028.
- Smykla J., Porazinskia D.L., Iakovenko N., Janko K., Weiner W.M., Niedbala W. & Drewnik M. 2010. Studies on Antarctic soil invertebrates: preliminary data on rotifers (Rotatoria), with notes on other taxa from Edmonson Point (northern Victoria Land, continental Antarctic). Acta Societatis Zoologicae Bohemicae 74, 135–140.
- Sohlenius B. & Boström S. 2005. The geographic distribution of metazoan microfauna on East Antarctic nunataks. Polar Biology 28, 439–448, doi: 10.1007/s00300-004-0708-z.
- Sohlenius B. & Boström S. 2008. Species diversity and random distribution of microfauna in extremely isolated habitable patches on Antarctic nunataks. Polar Biology 31, 817–825, doi: 10.1007/s00300-008-0420-5.
- Sohlenius B., Boström S. & Hirschfelder A. 1995. Nematodes, rotifers and tardigrades from nunataks in Dronning Maud Land, East Antarctica. Polar Biology 15, 51–56, doi: 10.1007/BF00236124.
- Sohlenius B., Boström S. & Hirschfelder A. 1996. Distribution patterns of microfauna (nematodes, rotifers and tardigrades) on nunataks in Dronning Maud Land, East Antarctica. Polar Biology 16, 191–200, doi: 10.1007/BF02329207.
- Sohlenius B., Boström S. & Jönsson K.I. 2004. Occurrence of nematodes, tardigrades and rotifers on ice-free areas in East Antarctica. Pedobiologia 48, 395–408, doi: 10.1016/j.pedobi.2004.06.001.
- Sugg P., Edwards J.S. & Baust J. 1983. Phenology and life history of Belgica antarctica, an Antarctic midge (Diptera: Chironomidae). Ecological Entomology 8, 105–113, doi: 10.1111/j.1365-2311.1983.tb00487.x.
- Suren A. 1990. Microfauna associated with algal mats in meltwater ponds of the Ross Ice Shelf. Polar Biology 10, 329–335, doi: 10.1007/BF00237819.
- van den Hoogen J., Geisen S., Routh D., Ferris H., Traunspurger W., Wardle D.A., de Goede R.G.M., Adams B.J., Ahmad W., Andriuzzi W.S., Bardgett R.D., Bonkoswki M., Campos-Herrera R., Cares J.E., Caruso T., Caixeta L.D., Chen X.Y., Costa S.R., Creamer R., Castro J.M.D., Dam M., Djigal D., Escuer M., Griffiths B.S., Gutierrez C., Hohberg K., Kalinkina D., Kardol P., Kergenteuil A., Korthals G., Krashevska V., Kudrin A.A., Li Q., Liang W.J., Magilton M., Marais M., Martin J.A.R., Matveeva E., Mayad E., Mulder C., Mullin P., Neilson R., Nguyen T.A.D., Nielsen U.N., Okada H., Rius J.E.P., Pan K., Peneva V., Pellissier L., da Silva J.C.P., Pitteloud C., Powers T.O., Powers K., Quist C.W., Rasmann S., Moreno S.S., Scheu S., Setala H., Sushchuk A., Tiunov A.V., Trap J., van der Putten W., Vestergard M., Villenave C., Waeyenberge L., Wall D.H., Wilschut R., Wright D.G., Yang J.I. & Crowther T.W. 2019. Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198, doi: 10.1038/s41586-019-1418-6.
- Velasco-Castrillón A., Gibson J.A.E. & Stevens M.I. 2014. A review of current Antarctic limno-terrestrial microfauna. Polar Biology 37, 1517–1531, doi: 10.1007/s00300-014-1544-4.
- Velasco-Castrillón A., Hawes I. & Stevens M. 2018. 100 years on: a re-evaluation of the first discovery of microfauna from Ross Island, Antarctica. Antarctic Science 30, 209–219, doi: 10.1017/S095410201800007X.
- Velasco-Castrillón A., McInnes S., Schultz M., Arróniz-Crespo M., D’Haese C., Gibson J., Adams B., Page T., Austin A., Cooper S. & Stevens M. 2015. Mitochondrial DNA analyses reveal widespread tardigrade diversity in Antarctica. Invertebrate Systematics 29, 578–590, doi: 10.1071/IS14019.
- Velasco-Castrillón A., Page T.J., Gibson J.A.E. & Stevens M. 2014. Surprisingly high levels of biodiversity and endemism amongst Antarctic rotifers uncovered with mitochondrial DNA. Biodiversity 15, 130–142, doi: 10.1080/14888386.2014.930717.
- Velasco-Castrillón A., Schultz M.B., Colombo F., Gibson J.A.E., Davies K.A., Austin A.D. & Stevens M.I. 2014. Distribution and diversity of soil microfauna from East Antarctica: assessing the link between biotic and abiotic factors. PLoS One 9, e87529, doi: 10.1371/journal.pone.0087529.
- Velasco-Castrillón A. & Stevens M.I. 2014. Morphological and molecular diversity at a regional scale: a step closer to understanding Antarctic nematode biogeography. Soil Biology and Biochemistry 70, 272–284, doi: 10.1016/j.soilbio.2013.12.016.
- Velázquez D., Jungblut A., Rochera C., Rico E., Camacho A. & Quesada A. 2017. Trophic interactions in microbial mats on Byers Peninsula, maritime Antarctica. Polar Biology 40, 1115–1126, doi: 10.1007/s00300-016-2039-2.
- Wall D.H., Adams B.J., Barrett J.E., Hopkins D.W. & Virginia R.A. 2006. A synthesis of soil biodiversity and ecosystem functioning in Victoria Land, Antarctica. Soil Biology and Biochemistry 38, 3001–3002, doi: 10.1016/j.soilbio.2006.04.018.
- Zawierucha K., Marshall C.J., Wharton D. & Janko K. 2019. A nematode in the mist: Scottnema lindsayae is the only soil metazoan in remote Antarctic deserts, at greater densities with altitude. Polar Research 38, article no. 3494, doi: 10.33265/polar.v38.3494.
- Zawierucha K., Porazinska D.L., Ficetola G.F., Ambrosini R., Baccalo G., Buda J., Ceballos J.L., Devetter M., Dial R., Franzetti, Fuglewicz U., Gielly L., Lokas E., Janko K., Jaromerska T.N., Koscinski A., Kozlowska A., Ono M., Parnikoza I., Pittino F., Poniecka E., Sommers P., Schmidt S.K., Shain D., Sikorska S., Uetake J. & Takeuchi N. 2021. A hole in the nematosphere: tardigrades and rotifers dominate the cryoconite hole environment, whereas nematodes are missing. Journal of Zoology 313, 18–36, doi: 10.1111/jzo.12832.
- Zawierucha K., Zmudczyńska-Skarbek K., Kaczmarek Ł. & Wojczulanis-Jakubas K. 2016. The influence of a seabird colony on abundance and species composition of water bears (Tardigrada) in Hornsund (Spitsbergen, Arctic). Polar Biology 39, 713–723, doi: 10.1007/s00300-015-1827-4.
- Zmudczyńska K., Olejniczak I., Zwolicki A., Iliszko L., Convey P. & Stempniewicz L. 2012. Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biology 35, 1233–1245, doi: 10.1007/s00300-012-1169-4.
- Zmudczyńska-Skarbek K., Barcikowski M., Drobniak S.M., Gwiazdowicz D.J., Richard P., Skubała P. & Stempniewicz L. 2017. Transfer of ornithogenic influence through different trophic levels of the Arctic terrestrial ecosystem of Bjørnøya (Bear Island), Svalbard. Soil Biology & Biochemistry 115, 475–489, doi: 10.1016/j.soilbio.2017.09.008.
- Zmudczyńska-Skarbek K., Zwolicki A., Convey P., Barcikowski M. & Stempniewicz L. 2015. Is ornithogenic fertilization important for collembolan communities in Arctic terrestrial ecosystems? Polar Research 34, article no. 25629, doi: 10.3402/polar.v34.25629.





