Health Benefits of Moringa oleifera


Опубликована Июнь 11, 2014
Последнее обновление статьи Дек. 26, 2022
Abstract
Phytomedicines are believed to have benefits over conventional drugs and are regaining interest in current research. Moringa oleifera is a multi-purpose herbal plant used as human food and an alternative for medicinal purposes worldwide. It has been identified by researchers as a plant with numerous health benefits including nutritional and medicinal advantages. Moringa oleifera contains essential amino acids, carotenoids in leaves, and components with nutraceutical properties, supporting the idea of using this plant as a nutritional supplement or constituent in food preparation. Some nutritional evaluation has been carried out in leaves and stems. An important factor that accounts for the medicinal uses of Moringa oleifera is its very wide range of vital antioxidants, antibiotics and nutrients including vitamins and minerals. Almost all parts from Moringa can be used ad a source for nutrition with other useful values. This mini-review elaborates on details of its health benefits.
Ключевые слова
Moringa oleifera, antioxidant, anti-microbial, anti-fibrotic, anti-inflammatory, anti-hyperglycemic
Introduction
Moringa (Moringa oleifera Lam), is a type of local medicinal Indian herb winch has turn out to be familiar in the tropical and subtropical countries. The other terms used for Moringa are Horseradish tree, Mulangay, Mlonge, Benzolive, Drumstick tree, Sajna, Kelor, Saijihan and Marango. Moringa oleifera is shown in scientific division to become from Kingdom: Plantae, Division: Magnoliphyta, Class: Magnoliopsida, Order: Brassicales, Family: Moringaceae, Genus: Moringa, Species: M. oleifera (Fahey, 2005).
Moringa oleifera is one of the vegetables of the Brassica order and belongs to tire family Moringaceae. The Moringaceae is a single genus family with 13 known species (Khawaja et al., 2010). Moringa oleifera is a small native tree of tire sub-Himalayan regions of North West India, winch is now indigenous to many regions in Africa, Arabia, South Fast Asia, the Pacific and Caribbean Islands and South America. Traditionally, besides being a daily used vegetable among people of these regions, the Moringa is also widely known and used for its health benefits. Among commoners, it has earned its name as ‘the miracle tree’ due to its amazing healing abilities for various ailments and even some chronic diseases. Several investigations were carried out to isolate bioactive compounds from various parts of the plant due to its various applications (Guevara et al., 1999). Therefore, herbal plants in medicine or known as phytomedicine are still trustworthy and widely applied as one of the alternative way in medicinal field due to its affordable cost (Abaiaka et al., 2009).
For centuries and in many cultures around tire world, tire medicinal usage of tire Moringa has been used to treat problems such as skin infections, anaemia, anxiety, asthma, blackheads, blood impurities, bronchitis, catarrh, chest congestion, cholera and many other illnesses (Khawaja et al., 2010; Hamza, 2010; Singh et al., 2012). Moringa oleifera also consists of anti -inflammatory, antispasmodic, anti-hypertensive, anti-tumour, anti-oxidant, antipyretic, anti-ulcer, anti-epileptic, diuretic, cholesterol lowering, renal, anti-diabetic, (Paliwal et al.,2011; Shanna et al., 2012) and hepatoprotective activities (Lai et al., 2010; Huang et al., 2012). It has also long been labelled for its great cosmetic value in which in recent years, tire Moringa has commonly been found to be used in various health care products including body and hair moisturisers and conditioners. It was also discovered that Moringa oil was used in skin ointments ever since the Egyptian times. The Moringa was claimed to be ‘the most nutrient-rich plant yet discovered’ by Khawaja et al. (2010).
Nutritional Composition
The Moringa’s incredible medicinal usage winch is claimed by many cultures and communities based on real-life experiences are now slowly being confirmed by science. Through research, the Moringa was found to contain many essential nutrients, for instance, vitamins, minerals, amino acids, beta-carotene, antioxidants, antiinflammatory nutrients and omega 3 and 6 fatty acids (Fahey, 2005; Hsu et al., 2006; Kasolo et al., 2010).
Nutrition content of a plant plays an essential function in medicinal, nutritional, and therapeutic properties (Al-Kharusi et al., 2009). It is believed that Moringa leave to consist high source of vitamin C, calcium, ß-carotene, potassium as well as protein. It works as an effective source of natural antioxidants. Due to the presence of several sorts of antioxidant compounds such as flavonoids, ascorbic acid, carotenoids, and phenolics, Moringa is able to extend the period of food containing fats (Dillard and German, 2000; Siddhuraju and Becker, 2003).
It was also found that each different part of the Moringa tree winch was studied, be it the fruits, seeds, leaves, flowers, bark and roots, all resulted in the discovery of at least one, or in most studies, a number of beneficial nutrients. It was similarly mentioned in an article by tire Trees For Life organization that ‘every part of tire Moringa tree is said to have beneficial properties that can serve humanity’.
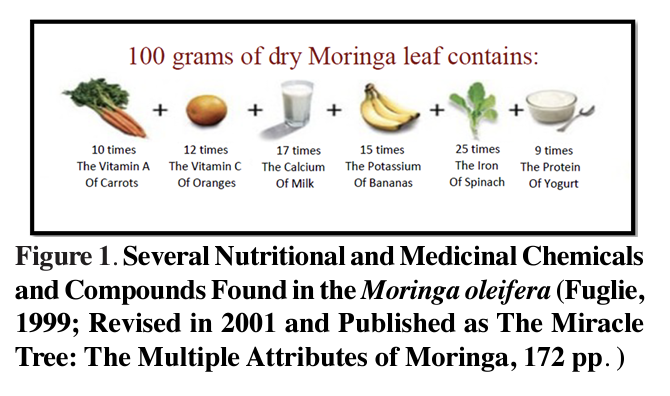
It is rare for a single plant to contain many essential nutrients and furthermore in high quantities. However, the Moringa on its own was reported to have a higher content of different nutrients compared to those found individually in several different types of food and vegetables (Figure 1). It was reported by several researchers at the Asian Vegetable Research and Development Centre (AVRDC) that the leaves of four of the Moringa species were rich in nutrients and antioxidants (Price, 2007) in which the nutrient content varied with a few factors such as preparation method, leaf age and harvest season. As commonly known, most vegetables lose their nutrients upon cooking. However, it was observed that Moringa leaves whether fresh, cooked or stored as dried powder for months without refrigeration, did not lose its nutritional value (Hsu et al., 2006). The leaves which were boiled resulted in three times more bio-available iron than tire raw leaves. These results were also seen in the powdered Moringa leaves.
In addition, the Moringa was found to have a group of unique compounds containing sugar and rhamnose, which are uncommon sugar-modified glucosinolates (Fahey et al., 2001; Fahey, 2005; Amaglo et al., 2010). These compounds were reported to demonstrate certain chemopreventive activity, by inducing apoptosis (Brunelli et al., 2010).
Anti-fibrotic/ulcer
Major contributors to the treatment of liver fibrosis discovered to date are natural drugs. Constant efforts and studies on these natural drugs to treat liver fibrosis are being carried out in search for effective anti-fibrotic agents. It was recently discovered that the Moringa oleifera seed extract exhibited anti-fibrotic effects on liver fibrosis in rats (Hamza, 2010). It showed significant protective effect against CC14-induced liver fibrosis in rats winch was confirmed by histological findings as well as biochemical analysis of a marker of collagen deposition in liver known as hydroxyproline. In the work by Hamza (2010), treatment with Moringa was found to stimulate hepatoprotective effects against hepatocellular injury by blocking tire increase of two serums, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), winch are indicators of liver health conditions.
In another study by Verma et al., (2012), tire effect of 50% ethanolic leaf extract of Moringa oleifera on pylorus ligation-induced, ethanol-induced, cold-restraint stress-induced and aspirin-induced gastric ulcers were investigated. The results of all these tests indicated that tire total ulcerogenic effect reduced, by showing a dosedependent anti-ulcerogenic activity by tire 50% ethanolic leaf extract. The extract was found to decrease acid-pepsin secretion as well as exhibit ulcer protective properties.
Anti-inflammatory Effects
Moringa has been practically used in medicinal field, throughout the decades to heal a huge amount of acute and chronic conditions. In vitro and in vivo studies with tire plant have recommended its effectiveness in treating inflammation, hyperlipidemia, and hyperglycemia (Bennett et al., 2003; Fahey, 2005; Mbikay, 2012). The properties of its phytochemicals, such as flavonols and phenolic acids were related to the anti-inflammatory, anti-oxidant and anti-bacterial activities (Mbikay, 2012).
Recently, tire explore for food components that triggers the inflammatory response has focused on particular attention due to confirmation linking chronic low-grade inflammation to insulin resistance and obesity. Some key biomarkers of inflammation have been identified as hallmark indicators of the pro-inflammatory response found in obesity-induced diabetes winch include cytokines: tumor necrosis factor alpha (TNFa), interleukin-1 beta (IL- 1b), interleukin-6 (IL-6), as well as inducible nitric oxide synthase (iNOS), and nitric oxide (NO), an important cellular signaling molecule in insulin signaling catalyzed by iNOS (Xu et al., 2003; Ferrante, 2007; Bhargava and Lee, 2012). iNOS expression and NO overproduction have been implicated in tire pathogenesis of disease states, particularly related with chronic inflammation (Hobbs et al., 1999).
It has been discovered that hepatic myeloperoxidase activity can be carried out as a marker of inflammation and tissue neutrophil accumulation and activation (Hillefass et al., 1990). In a study by Caceres et al. (1992), infused Moringa oleifera seeds and roots showed inhibition of carrageenan-induced hind paw edema. The inhibition by tire seed infusion conversely was dose-dependent as compared to the root infusion winch showed inactivity at certain dosages. However, only seed infusion was stated to be worthy of further study due to indications of more convincing anti -inflammatory inhibition.
The hepatoprotective properties of Moringa seed extract which was discovered from the anti-fibrotic study by Hamza (2010) indicated that tire Moringa also possessed anti-inflammatory properties against CC14- induced liver damage and fibrosis. This finding was confirmed by the decrease of globulin level in serum and tire myeloperoxidase activity in liver. Additionally, in tire histopathological analysis, a decrease in inflammatory cells infiltrations was discovered.
Antimicrobial Effects of Moringa
The assorted extracts of Moringa’s morphological parts such as seeds cotyledon, seeds’ coat, stem bark, leaves, root bark are reported to possess antimicrobial potential (Arora et al., 2013). Recently, Onsare et al. (2013) have reported preliminary work on tire antimicrobial activity of aqueous extract of pods’ husks against Gram positive, Gram negative pathogenic bacteria and yeast strains.
In a study by Singh et al. (2012), the antimicrobial activity of Moringa oleifera was examined using the main model Kirby-bauer disc diffusion method in winch 50% of ethanolic Moringa leaf extract was used. The results showed that tire 50% ethanolic extract successfully displayed anti-bacterial activity however only little. Even at higher concentrations, the extract displayed mild inhibitory activity and no activity at all against pseudomonas.
Peixoto et al. (2011) reported that in their study, tire aqueous and ethanolic Moringa leaf extracts indicated promising potential as a treatment for certain bacterial infections. The antibacterial activity of the Moringa extract was observed to be greater against gram-positive species (8. aureus and E. faecalis) than against grampositive species (E. coli, Salmonella, P. aeruginosa, V. parahaemolyticus and A. caviae) which was also indicated in several other studies (Grosvenor et al., 1995; Kudi et al„ 1999; Awadh et al„ 2001).
Anti-hyperglycemic of Moringa
Diabetes Mellitus (DM) is a chronic metabolic disorder. Diabetic patients exhibit a stage of chronic hyperglycemia and glucose tolerance impairment (Tiwari and Roa, 2002). Moringa oleifera is well known for its pharmacological actions and is used for the traditional treatment of diabetes mellitus (Bhishagratna, 1991; Babu and Chaudhuri, 2005).
The anti-diabetic effects of some medicinal plant were strengthened by scientific data as herbal remedies for diabetes are recognized in different societies (Grove and Altman, 2002). Ajit et al., 2003 reported that hypoglycemic activity of Moringa oleifera, with significant blood glucose lowering activities has been confirmed. Methanol extract of its dried fruit powder has produced N-Benzyl thiocarbamates, N-benzyl carbamates, benzyl nitriles and a benzyl; winch prove to trigger insulin release significantly from tire rodent pancreatic beta cells, and have cycloxygenase enzyme and lipid peroxidation inhibitory activities (Francis et al., 2004).
Hypoglycemic and anti-hyperglycemic activity of tire leaves of Moringa oleifera may be probably due to tire presence of terpenoids, winch appears to be involved in tire stimulation of tire ß-cells and tire subsequent secretion of preformed insulin (Tende et al., 2011).
Antioxidant Properties of Moringa
Naturally occurring antioxidants, particularly polyphenols, are the main plant compounds that are able to decrease oxidative damage in tissues by indirect enhancement of a cell or by free radical scavenging (Du et al., 2010). The leaves of the Moringa oleifera tree have been reported to demonstrate antioxidant activity dues to its high amount of polyphenols (Sreelatha and Padma, 2009; Verma et al., 2009). Moringa oleifera extracts of both mature and tender leaves exhibit strong antioxidant activity against free radicals, prevent oxidative damage to major biomolecules and give significant protection against oxidative damage (Sreelatha and Padma, 2009).
A comparative study indicated that mature Moringa oleifera leaf extract exhibited better values of enzymatic and non-enzymatic antioxidants. In tire DPPH (2,2-Diphenyl- 1-Picrylhydrazyl) free radical scavenging activity test, both mature and tender leaf extracts showed significant reduction of DPPH radicals. The scavenging activity was suggested to be attributed to its hydrogen donating ability and was seen more in the mature leaf extract (Sreelatha and Padma, 2009).
A further TEG (Thin layer chromatography) analysis was conducted to identify the chemical nature of active compounds which were possibly providing these antioxidant protection properties. According to Sreelatha and Padma (2009), qualitative analysis of the extracts revealed the presence of phenolics, flavonoids and trace amounts of alkaloids, in both mature and tender leaves.
Similarly, another study by Siddhuraju and Becker (2003) using the same DPPH assay to determine the antioxidant activity, revealed that tire extracts of Moringa leaf samples from three different agroclimatic origins had very high radical scavenging activity. It was stated that generally, the higher the total polyphenols, the higher tire antioxidant activity, in winch it is most likely to be due to the combined action of several existing substances as well as the high hydrogen donating ability. In addition, tins study also used several other methods to assess tire antioxidant activity of the obtained Moringa extracts, whereby all other methods likewise demonstrated the same antioxidant activity in terms of reducing potassium ferricyanide, scavenging superoxide radicals, preventing peroxidation of lipid membranes in liposomes, inhibiting oxidation of microsomes in rat liver, inhibiting the peroxidation of linoleic acids and preventing bleaching of carotenes.
The antioxidant properties of the Moringa oleifera was also examined by Verma et al. (2012) whereby 50% ethanolic leaf extract was tested to study tire lipid peroxidation (LPO), catalase (CAT) and superoxide dismutase (SOD) activities. The antioxidant properties of tire Moringa extract was found to change in SOD, CAT and LPO levels in rat gastric mucosa. There was a reported increase in gastric mucosal SOD and LPO activities during ulcer conditions winch indicated an antioxidant defence Ahmad Faizal Abdull Razis et al mechanism by the Moringa oleifera extract (Verma et al., 2012).
In another study, goats meat which was supplemented with Moringa oleifera leaves and several other natural products, was tested for its antioxidant potential starting off with the total phenol content (TPC) estimation then followed by the DPPH, 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic diammonium salt) (ABTS), glutathione, lipid peroxidation, catalase and superoxide dismutase (SOD) methods. The nutritional and fatty acid profiles of the Moringa oleifera supplemented goats meat indicated highest antioxidant activity compared to the goats meat supplemented with the other natural products (Qwele et al., 2013). The antioxidant activity was also implied by the high concentration of total phenol content as well as the reducing power of the Moringa oleifera supplemented meat. In addition, compared to the other natural products, the meat supplemented with Moringa oleifera leaves exhibited the highest efficiency (93. 51%) in terms of radical scavenging and highest increase (93. 13%) in SOD activity (Qwele et al., 2013).
Anti-tumour Properties of Moringa
A study to isolate several bioactive compounds from the Philippine grown Moringa oleifera Lam. to examine the anti-genotoxic and anti-inflammatory activities, also reported the effect of several isolates as anti-tumour promoters. Guevara et al. (1999) presented evidence on the function of mainly one of these bioactive compounds, niazimicin, as an inhibitor against the two-stage mouse tumourigenesis. The results from in vitro screening suggested that several of the test compounds, particularly 4-(ct-L-rhamnosyloxy) benzyl isothiocyanate, niazimicin and ß-sitosterol-3-O-ß-D-glucopyranoside were strong anti-tumour promoters. Whilst in the in vivo two-stage carcinogenesis test on mouse skin, niazimicin exhibited 50% delay in the promotion of tumours and decreased the incidence of papilloma bearing mice by 80% at 10 weeks and 17% at 20 weeks of promotion (Guevera et al., 1999). This study concluded that niazimicin was a potent antitumour promoter in chemical carcinogenesis.
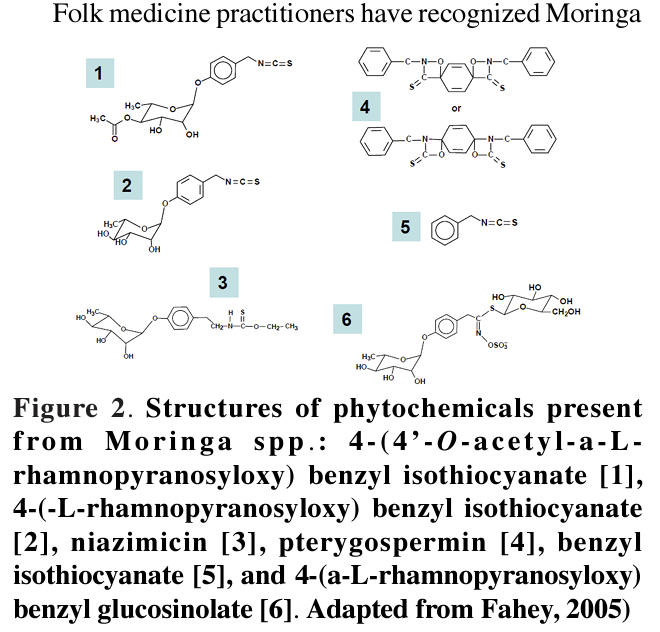
species to having value in tumour therapy (Hartwell, 1971), anticancer potential were detected in compounds [1] and [2] (Fahey et al., 2004). Recently, compound [1] and the correlated compound [3] have become as dominant inhibitors of phorbol ester (TPA)-induced Epstein-Barr virus initial activation of antigen in lymphoblastoid (Burkitt’s lymphoma) cells (Guevara et al., 1999; Murakami et al., 1998). Tumour promotion is also inhibited by compound [3] in a mouse two- stage DMBA (7, 12-Dimethylbenz(a)anthracene)-TPA (12-O-tetradecanoylphorbol-13-acetate) tumour model in one of these studies (Murakami et al., 1998) as shown in Figure 2. Bharali and colleagues (2003) reported skin tumour prevention subsequent to ingestion of drumstick (Moringa seedpod) extracts.
Anti-cancer Properties of Moringa
Moringa is revealed to possess potential therapeutic effects to light cancer, rheumatoid arthritis, diabetes, and some other ailments. Particularly in South Asia, it works as treatment for different diseases in the indigenous system of medicine (Mehta et al., 2003; Karadi et al., 2007; Roy et al., 2007).
In a recent study by Budda et al. (2011), it was stated that Moringa oleifera Lam pod could be a potential chemopreventive agent. The dose dependent administration of boiled Moringa oleifera (bMO) caused the incidence and multiplicity of tumours to decrease especially at the highest dose (6.0%) of bMO. It was further reported that when compared to the lower bMO doses, the number of tubular adenocarcinomas reduced in correspondence to the number of superficial adenocarcinomas.
Budda and his team (2011) stated that the presence of fatty acids could have attributed to the chemopreventive effect of bMO which modulates apoptosis in colon carcinogenesis. In addition, the presence of niazimicin and glucomoringin which have been reported to inhibit tumour cell proliferation, were also mentioned as possible compounds contributing to the anti-colon carcinogenic effects of bMO. For the effect of bMO on several protein expressions, it was reported that in a dose dependent manner, all three PCNA, iNOS and COX-2 gene expressions were down-regulated which concluded the chemopreventive effect of bMO.
A balance as well as the induction of Phase I and II drug metabolising enzymes is well known to indicate a defence against chemical carcinogens (Singh et al., 2000). In 2003, Bharali et al. revealed in their study that the hydroalcoholic Moringa oleifera extract works as a bifunctional inducer, inducing both Phase-1 and Phase-11 enzymes. It was reported to have improved the levels of hepatic cytochrome b5, cytochrome P450 and gluthione-S- transferase (GST). A similar study by Sharma et al. (2012) also reported increased levels of the cytochrome P450 and cytochrome b5 by Moringa oleifera pod extract. The cytochrome P450 was found to functioned as a blocking agent (Sharma et al., 2012; Bharali et al., 2003) in the Phase II metabolism whereas the increased levels of GST by the Moringa oleifera extract was reported to have possibly been the major indication of chemoprevention properties (Bharali et al., 2003).
The study by Shanna et al. (2012) reported that the enzyme (glutathione and GST) activity loss was restored by the Moringa pod extract in which diese enzymes offer a major protection role against the effects of carcinogens. Tins was supported by a previous study by Singh et al., (2000) stating the protection role of GST. The antioxidant properties of the Moringa oleifera that relates closely to its potential as a chemopreventive agent was confirmed through phytochemical screening of the pod extract (Paliwal et al., 2011; Shanna et al., 2012). Together with tins, the hepatoprotective effect Moringa oleifera through the restoration of aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP) was also testified (Shanna et al., 2012).
In another study by Shanna and Paliwal (2012), to investigate more deeply the chemopreventive effects of Moringa oleifera pod extract, it was reported that the oxidative damage induced by the PAH 7, 12-dimethylbenz(a) anthracene (DMBA), can be prevented by die Moringa extract as well as its isolated saponin. These findings suggested that Moringa oleifera shows chemopreventive effects by increasing the antioxidant levels and reducing die free radical formation.
Against several different cancer cell lines (lung, liver, colon and neuroblastoma), the Moringa oleifera seed extract demonstrated selective growth inhibition, reaching up to 95% inhibition towards the neuroblastoma cell line (Shaban et al., 2012). Earlier in 2010, Purwal et al. reported that tumours treated with methanolic extracts of Moringa fruits and leaves showed slow growth which indicates effective deterioration of tumours. The most effective dosages of the extracts in terms of volume doubling time and growth delay, which both indicate tumour inhibitory property, were observed at 500mg/kg.
Anti-clastogenic Properties of Moringa
In recent years, there has been a new interest in die clastogenicity and anti-clastogenicity of Moringa oleifera pod in establishing its health benefits. The results from a study by Promkum et al. (2010) showed that bMO did not possess any clastogenic activity in mice upon consuming a diet consisting of 1.5%, 3.0% and 6.0% bMO. The Moringa oleifera demonstrated free radical scavenging properties that directly indicate anti-clastogenic effects winch was found to be due to its rich vitamin C content. The anti-clastogeniticity test in tins study showed activity against both direct mitomycin С (MMC) and indirect- acting DMBA clastogens. It was finally concluded dial bMO at 2. 1, 4. 3 and 8. 5g/kg BW doses did not show clastogenic effects whilst its anti-clastogenic potential is modulated by the direct acting carcinogenesis process.
Conclusion
In conclusion, it is proven in numerous cases that tile Moringa oleifera tree possesses a wide range of medicinal and therapeutic properties. For instance, in tins paper, it views the general nutrition contents of the Moringa Health Benefits of Moringa oleifera up to several specific remedial properties including its anti-fibrotic, anti-inflammatory, anti-microbial, anti- hyperglycemic, antioxidant, anti-tumour and anti-cancer properties. Further studies for the mechanism of action and constituents of the Moringa plant may provide incredible capabilities to develop pharmacological products. The further studies should emphasis on probable mode of action of the isolates and possible structural-activity relationship as the chemical constituents of Moringa oleifera are very well investigated and documented. In conclusion, Moringa oleifera has numerous applications in medicinal field.
References
- Abalaka ME, Olonitola OS, Onaolapo JA, et al (2009). Evaluation of acute toxicity Momordica charantia extract, using wistar rats to determine safety level and usefulness of the plant ethnochemotheraphy. Int J Appl Sci, 3, 1-6.
- Ajit K, Choudhary BK, Bandyopadhyay NG (2002). Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol, 84, 105-8.
- Al-Kharusi LM, Elmardi MO, Ali A, et al (2009). Effect of mineral and organic fertilizers on the chemical characteristics and quality of date fruits. Int J Agri Biol, 11, 290-6.
- Amaglo NK, Bennet RN, Curto RBL, et al (2010). Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. J of Food Chem, 122, 1047-54. https://doi.org/10.1016/j.foodchem.2010.03.073
- Arora DS, Onsare JM, Kuar H(2013). Bioprospecting of Moringa (Moringaceae):microbiological perspective. J Pharmacog Phytochem, 1, 193-215.
- Awadh A, Julich WD, Kusnick C, et al (2001). Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities, J Ethnopharmacol, 74, 173-9. https://doi.org/10.1016/S0378-8741(00)00364-0
- Babu R, Chaudhuri, M. (2005). Home water treatment by direct filtration with natural coagulant. J Water Health, 3, 27-30.
- Bennett RN, Mellon FA, Foidl N, et al (2003). Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J Agri Food Chem, 51, 3546-53. https://doi.org/10.1021/jf0211480
- Bharali R, Tabassum J, Azad MRH (2003). Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pacific J Cancer Prev, 4, 131-139.
- Bhargava P, Lee C(2012). Role and function of macrophages in the metabolicsyndrome. Biochem J, 442, 253-62. https://doi.org/10.1042/BJ20111708
- Bhishagratna KK (1991). An English translation of SushrutamSamhita based on the original Sanskrit text, Chowkhamba Sanskrit Series, Varanasi, India,3, 213-9.
- Brunelli D, Tavecchio M, Falcioni C, et al (2010). The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem Pharmacol, 79, 1141-8. https://doi.org/10.1016/j.bcp.2009.12.008
- Budda S, Butryee C, Tuntipopipat S, et al (2011). Suppressive effects of Moringa oleifera Lam pod against mouse colon carcinogenesis induced by azoxymethane and dextran sodium sulphate. Asian Pacific J Cancer Prev, 12, 3221-8.
- Caceres A, Saravia A, Rizzo S, et al (1992). Pharmacologic properties of Moringa oleifera. 2: Screening for antispasmodic, anti-inflammatory and diuretic activity, J Ethnopharmacol, 36, 233-7. https://doi.org/10.1016/0378-8741(92)90049-W
- Dillard CJ, German JB (2000). Review Phytochemicals: nutraceuticals and human health. J Sci Food Agric, 80, 1744-6. https://doi.org/10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W
- Fahey JW, Zalcmann AT, Talalay P (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochem. 56, 5-51. https://doi.org/10.1016/S0031-9422(00)00316-2
- Fahey JW, Dinkova-Kostova AT, P. Talalay. The "Prochaska" microtiter plate bioassay for inducers of NQO1. Methods in Enzymology, 382, 243-58.
- Fahey, JW (2005). Moringa oleifera: A review of the medicinal evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J, 1, 5.
- Ferrante, AW (2007). Obesity-induced inflammation: a metabolic dialogue in the lanugage of inflammation. J Intern Med, 262, 408-14. https://doi.org/10.1111/j.1365-2796.2007.01852.x
- Francis JA, Jayaprakasam B, Olson LK, et al (2004). Insulin secretagogues from Moringa oleifera with cyclooxygenase enzyme and lipid peroxidation inhibiting, Helvitica Chimica Acta, 87, 317-26. https://doi.org/10.1002/hlca.200490029
- Fuglie LJ (1999). The Miracle Tree: Moringa oleifera: Natural Nutrition for the Tropics. Church World Service, Dakar. pp. 68; revised in 2001 and published as The Miracle Tree: The Multiple Attributes of Moringa, pp. 172
- Grosvenor PW, Gothard PK, William MC,et al (1995). Medicinal plants from Riau province, Sumatra, Indonesia. J Ethnopharmacol, 45, 75-95. https://doi.org/10.1016/0378-8741(94)01209-I
- Grove JK, Altman WM (2002). Medicinal Plants of India with antidiabetic potential. J Ethnopharmacol, 81, 81-100. https://doi.org/10.1016/S0378-8741(02)00059-4
- Guevara AP, Vargas C, Sakurai H, et al (1999). An antitumour promoter from Moringa oleifera Lam. Mutat Res, 440, 181-8. https://doi.org/10.1016/S1383-5718(99)00025-X
- Hamza AA(2010). Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol, 48, 345-55. https://doi.org/10.1016/j.fct.2009.10.022
- Hillefass LM, Riswold DE, Brickson B, et al (1990). Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods, 24, 285-95. https://doi.org/10.1016/0160-5402(90)90013-B
- Hobbs AJ, Higgs A, Moncada S(1999). Inhibition of nitric oxide synthase as apotential therapeutic target. Annu Rev Pharmacol Toxicol, 39, 191-220. https://doi.org/10.1146/annurev.pharmtox.39.1.191
- Hsu R, Midcap S, Arbainsyah DWL(2006). Moringa oleifera: Medicinal and Socio-Economical Uses. Internationa Course on Economic Botany, National Herbarium Leiden, the Netherlands.
- Huang GJ, Deng JS, Huang SS, et al (2012). Protective effect of antrosterol from Antrodia camphorata submerged whole broth against carbon tetrachloride-induced acute liver injury in mice. Food Chem, 132, 709-16. https://doi.org/10.1016/j.foodchem.2011.11.004
- Kasolo JN, Bimenya GS, Ojok L, et al (2010). Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res, 4, 753-7.
- Khawaja TM, Tahira M, Ikram UK (2010). Moringa oleifera: a natural gift - A review. J Pharm Sci Res, 2, 775-81.
- Kudi AC, Uhoh JU, Eduvie LO, (1999). Screening of some Nigerian medicinal plants for antibacterial activity. J Ethnopharmacol, 67, 225-8 https://doi.org/10.1016/S0378-8741(98)00214-1
- Lai TY, Weng YJ, Kuoi WW (2010). Taohe Chengqi Tang ameliorates acute liver injury induced by carbon tetrachloride in rats. J Chin Integr Med, 8, 49-55.
- Mbikay M, (2012). Therapeutic potential of Moringa oleifera leaves in chronichyperglycemia and dyslipidemia: a review. Front Pharmacol, 3, 1-12.
- Onsare, JG, Kaur, H, Arora, DS (2013). Antimicrobial activity of Moringa oleifera from different locations against some human pathogens. J Med Plants, 1, 80-91.
- Paliwal R, Sharma V, Pracheta S, et al (2011). Antinephrotoxic effect of administration of Moringa oleifera Lam in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice, Biol Med, 3, 27-35.
- Paliwal R, Sharma V, Pracheta, Sadhna S (2011). Elucidation of free radical scavenging and antioxidant activity of aqueous and hydro-ethanolic extracts of Moringa oleifera pods. Res J Pharmacy Technology, 4, 566-571.
- JR, Silva GC, Costa RA, et al (2011). In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med, 4, 201-4. https://doi.org/10.1016/S1995-7645(11)60069-2
- Price ML, (2007). The Moringa Tree. ECHO Technical Note. North Fort Myers, USA.
- Promkum C, Kupradinun P, Tuntipopipat S, Butryee C (2010). Nutritive evaluation and effect of Moringa oleifera pod on clastogenic potential in the mouse. Asian Pacific J Cancer Prev, 11, 627-32.
- Purwal L, Pathak AK, Jain UK (2010). In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline, 1, 655-65.
- Shaban A, Mishra GM, Nautiyal R, Srivastava S, Tripathi K, Chaudhary P, Verma SK (2012). In vitro cytotoxicity of Moringa oleifera against different human cancer cell lines. Asian J Pharm Clinical Res, 5, 271-2.
- Sharma V, Paliwal R, Janmeda P, Sharma S (2012). Renoprotective effects of Moringa oleifera pods in 7, 12 dimethylbenz[a] anthracene exposed mice, J Chin Int Med, 10, 1171-8.
- Sharma V, Paliwal R, Janmeda P, Sharma S (2012). Chemopreventive efficacy of Moringa oleifera pods against 7, 12-dimethylbenz[a]anthracene induced hepatic carcinogenesis in mice. Asian Pacific J Cancer Prev, 13, 2563-9. https://doi.org/10.7314/APJCP.2012.13.6.2563
- Siddhuraju P, and Becker K (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam. ) leaves. J Agri Food Chem, 51, 2144-5. https://doi.org/10.1021/jf020444+
- Singh GP, Sharma SK(2012). Antimicrobial evaluation of leaf extract of Moringa oleifera Lam. Int Res J Pharm, 3, 1-4.
- Singh RP, Padmanathi B, Rao AR (2000). Modulatory influence of Adhatoda vesica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism antioxidant stats and lipid peroxidation in mice. Molec Cellular Biochem 213, 99-109. https://doi.org/10.1023/A:1007182913931
- Tende JA, Ezekiel I, Dikko AAU (2011). Effect of ethanolic leaves extract of Moringa oleifera on blood glucose levels of streptozocin-induced diabetics and normoglycemic Wistar rats. British J Pharmacol Toxicol, 2, 1-4.
- Tiwari AK, Roa M (2002). Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Current Sci, 83, 30-8.
- Velasco P, Francisco M, Cartea ME (2011). In: Ronald Ross Watson and Victor R. Preedy (Eds. ), Bioactive Foods and Extracts: Cancer Treatment and Prevention. CRC Press, Boca Raton, 3-29.
- Verma AR, Vijayakumar M, Mathela CS, et al (2009). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food and Chem Toxicol, 47, 2196-201. https://doi.org/10.1016/j.fct.2009.06.005
- Verma VK, Singh N, Saxena P, et al (2012). Anti-ulcer and antioxidant activity of Moringa oleifera (Lam) leaves against aspirin and ethanol induced gastric ulcer in rats, Int Res J Pharm, 2, 46-7.
- Xu H, Barnes GT, Yang, Q (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest, 112, 1821-30. https://doi.org/10.1172/JCI200319451





