In search of the “I”: Neuropsychology of lateralized thinking meets Dynamic Causal Modeling


Опубликована Июль 1, 2017
Последнее обновление статьи Дек. 21, 2022
Abstract
Background. Ideas about relationships between “I”, egocentric spatial orientation and the sense of bodily “Self ” date back to work by classics of philosophy and psychology. Cognitive neuroscience has provided knowledge about brain areas involved in self-referential processing, such as the rostral prefrontal, temporal and parietal cortices, often active as part of the default mode network (DMN).
Objective and Method. Little is known about the contribution of inferior parietal areas to self-referential processing. Therefore, we collected observations of everyday behavior, social communication and problem solving in patients with brain lesions localized either in the left inferior parietal cortex (LIPC group, n = 45) or the right inferior parietal cortex (RIPC group, n = 58).
Results. A key characteristic of the LIPC group was an overestimation of task complexity. This led to a prolonged phase of redundant and disruptive contemplations preceding task solution. In the RIPC group, we observed disorders in reflective control and voluntary regulation of behavior. Abilities for experiencing emotions, understanding mental states, and social communication were to a great extent lost. Results are interpreted within a multilevel framework of cognitive-affective organization (velichkovsky, 2002). In particular, we highlight the role of right-hemisphere mechanisms in self-referential cognition, emotional and corporeal awareness. This is consistent with recent data on a profound asymmetry in connectivity of left and right hippocampi within the DMN (Ushakov et al., 2016)
Conclusion. It seems that the center of egocentric spatial representation plays a special role in accessing self-related data. Normally, the right hippocampus provides a holistic representation of surrounding and, thus, an easy-to-find gateway into much of what we used to call “subjective experience”. This heuristics becomes misleading in the case of right-sided brain lesions.
Ключевые слова
Egocentric spatial orientation, Self-referential cognition, levels of cognitive organization, emotions, lateralization, dynamic causal modeling (DCM), hippocampal formation, thinking, neuropsychology
Introduction
Thinking in patients with brain damages of different etiology remains a relatively weakly studied chapter of cognitive neuropsychology, both from point of view of diagnostics and that of rehabilitation. Despite a substantial number of diagnostic tests and an abundance of fractional data, the overall picture of this central neurocognitive issue still is fragmented and contradictory. The lack of a conceptual Gestalt makes it difficult to elucidate factors influencing learnability and find the ways to a better social adaptation of patients. Classical research devoted to the analysis of cognitive impairments in solution of arithmetic tasks (Luria, & Tsvetkova, 1966), verbal-logical inferences (Balonov, Deglin, & Tschernigovskaja, 1979), or visual-spatial constructive tests (Khomskaja, 1987; Korsakova, & Moskovichute, 1988) paid relatively little attention to the everyday problems of patients, though such problems are a particularly importance source of data for cognitive conceptualization. With a few exceptions, studies of the modern neuroimaging era demonstrate even less interest to the modeling of everyday tasks and situations (see, e.g., Gazzaniga, 2009).
In the present article, we attempted to combine a more phenomenological approach, which takes into account everyday problems of patients suffering from unilateral brain lesions, with new knowledge about human brain structural, functional and in particular effective (cause-and-effect) connectivity. In one such development, a recent diffusion tensor imaging (DTI) analysis of anatomical white matter asymmetries across the whole brain of 41 children and adolescents with Autism Spectrum Disorders (ASD) and a matched control group of 44 typically developing (TD) participants revealed that children with ASD have reduced lateralization compared to TD children who showed significant asymmetry with rightward anisotropy (Carper, Treiber, DeJesus, & Müller, 2016). These findings can be interpreted as reflecting different processing modes in two hemispheres. The “division of labor” between hemispheres appears to be diminished in ASD, possibly underlying the characteristic pattern of this group’s deficiency in social intelligence.
In another line of research, systematic hemispheric differences in molecular mechanisms were discovered even for closely located brain regions, such as the frontopolar Brodmann Areas 10 on the left (BA10L) and on the right (BA10R). Similarly, this research shows that most of the strongly expressed genes – and almost all of the differentially expressed protein-encoding genes -- were detected in the right frontopolar cortex (Dolina et al., 2017). A neuropsychological pendant to these data is the well-established knowledge that if the left hemisphere supports basic linguistic functions, the right prefrontal cortex might be important for understanding of metaphorical language, humor, irony and sarcasm (Balonov, Deglin, & Tschernigovskaja, 1985; Krotkova, & Velichkovsky, 2008; Shammi, & Stuss, 1999). In addition, right prefrontal areas are mainly involved in processes of autobiographical memory and personal planning for the future (Dickerson, & Eichenbaum, 2010). Both these groups of processes are directly related to our subjective experience, i.e., to our conscious «Self».
The picture of prefrontal (likely right-sided) involvement in the higher-order metacognitive processing is appealing and receiving support (Craik et al., 1999; Sokolov, 2013; Stuss, Rosenbaum, Malcom, Christiana, & Keenan, 2005; Velichkovsky, Klemm, Dettmar, & Volke, 1996) but it may be incomplete. There is another region seemingly realizing similar functions with respect to self-referential cognition, e.g. as related to retrieval of autobiographical memories and personal planning for the future. The region includes the inferior parietal lobe and the temporoparietal junction. Thereafter, we will call these ‘left and right inferior parietal cortex’ (LIPC and RIPC, respectively). Basically, these are structures of higher-order multimodal sensory integration, whereby LIPS is responsible for representation of the right and RIPS for representation of the left side of the surrounding.
One reason for the increased interest in this region is its role in the default mode network (DMN), a set of interconnected brain areas that are activated in the resting state and deactivated by any cognitively effortful task (Arsalidou, Pascual‐Leone, Johnson, Morris, & Taylor, 2013; Gusnard, Akbudak, Shulman, & Raichle, 2001; Raichle et al., 2001). Hypotheses on the DMN functionality have also been formulated mostly relating it to higher-order aspects of consciousness and cognition (Gusnard, & Raichle, 2001; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008). The main parts of the DMN have been identified in the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), and the inferior parietal cortex of both hemispheres (Buckner, Andrews-Hanna, & Schacter, 2008). With respect to hippocampal formation, rank correlations of activity also reveal the pattern of activation/deactivation characteristic of the DMN (Vincent, Bloomer, Hinson, & Bergmann, 2006). Connectivity patterns of hippocampal formation are of particular interest because of its crucial role in episodic memory processes (Dickerson, & Eichenbaum, 2010) and in representation of surrounding space (Burgess, Jackson, Hartley, & O’Keefe, 2000; Moser, & Moser, 2008).
Functional connectivity of both hippocampi has been analyzed extensively, for instance, in a recent meta-analytic study by Robinson, Salibi and Deshpande (2016). However, functional data have a low scientific status, as they are only correlational in nature. Therefore, of importance are studies where effective (cause-and-effect) relations among left and right hippocampal formation (LHIP and RHIP, respectively) and other DMN structures have been for the first time investigated by a combination of the functional magnetic resonance imaging (fMRI) and the mathematical method of spectral dynamic causal modeling (DCM). The method’s main idea is to evaluate parameters of a biologically-validated model of the neuronal system so that it could predict the observed fMRI data in the best way (Sharaev, Zavyalova, Ushakov, Kartashov, & Velichkovsky, 2016; Ushakov et al., 2016). These studies conducted on a group of 30 healthy right-handed subjects led us to the discovery of a profound asymmetry in LHIP and RHIP effective connections. Fig.1 illustrates this asymmetric pattern of interactions. LHIP demonstrated a high involvement in the DMN activity, with information outflow preponderant to all other DMN regions including RHIP, as shown by our analysis of two 5-nodes and one 6-nodes interactions. Causal interactions of LHIP with inferior parietal cortex were bidirectional only in the case of LIPC: there was not inflow to LHIP from RIPC. This means that in terms of spatial representation LHIP had access to information only about contralateral, right hemispace. On the contrary, RHIP was affected by inputs from both LIPC and RIPC that would allow a holistic – left and right-sided – multimodal representation of egocentric space (for a detailed analysis of the models, see Ushakov et al., 2016).
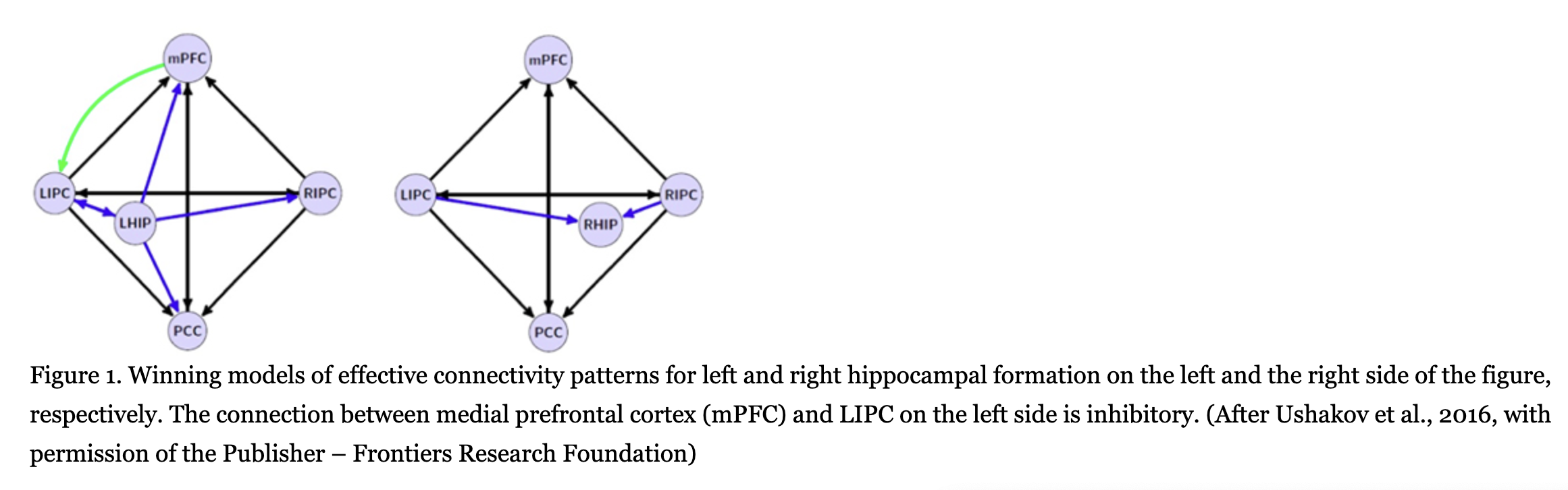
In our view, this pattern of asymmetry in effective connections of the hippocampal regions may be related to lateralization phenomena in verbal and spatial domains known in human neurophysiology, neuropsychology, and neurolinguistics1. As a matter of fact, there is an obvious drawback of such lateralized architecture: a destruction of RHIP or RIPC could lead to the left-sided spatial hemi-neglect not compensated by preserved LHIP/LIPC interconnections. This phenomenon is well-known from clinical data (Harrison, 2015; Howard, & Templeton, 1966; Luria, 1966). Distortions of corporeal awareness such as out-of-body experience (Blanke, & Mohr, 2005), asomatognosia (Baier, & Karnath, 2008) and anosognosia (Heilman, 2014; Vallar, Bottini, & Sterzi, 2003) have also been described with the same locus of lesions in the posterior part of the right hemisphere. Lateralization of higher-order cognitive and emotional processes in patients with local brain damages to either of LIPC or RIPC is by far less investigated though it could be supposed in light of previous observations (Krotkova, & Velichkovsky, 2008; Singh-Curry, & Husain, 2009). Comparative analysis of these processes in the relevant groups of patients was the primary objective of this study.
Method
Clinical material
We based this study on descriptions including more than 270 clinical cases of patients with unilateral brain damages of different etiology (e.g., traumata, tumors and blood vessel dysfunctions). Over several years, all the patients were observed in their everyday behavior and went through neuropsychological intervention programs in the division of rehabilitation at the Institute of Neurosurgery named after academician N.N. Burdenko in Moscow. From this database, we excluded cases in the following two categories: first, patients with relatively narrow lesions of primarily and secondary sensory regions (according to well-known neurological criteria – see Kolb, & Whishaw, 2015; Luria, 1966); second, patients with substantial damages of prefrontal regions. These latter damages would create specific difficulties for neuropsychological analysis due to diverse manifestations of dysexecutive (“frontal-lobe”) syndrome such as instability of attention, lack of motivation and general inactivity. The main reason for the exclusion was however our intention to select a target group of patients with lesions in the posterior tertiary regions of cortex overlapping with the loci of LIPC and RIPC to be consistent with data on effective connections within DMN network.
As a result, two samples of patients with corresponding localization of brain damages were selected for a close analysis of their everyday behavior: 45 cases (24 females), mean age 48 years, with left hemisphere localization and 58 cases (31 females), mean age 45 years, with lesions in the right hemisphere, all around inferior parietal lobe and temporoparietal junction. According to well-known classification of Brodmann cytoarchetechtonic maps (e.g. Kolb, & Whishaw, 2015), lesions included BA 39. Often, neighboring areas BA22, 37, and 40 were also part of the lesion. This localization, which roughly corresponded to the definition of LIPC and RIPC in the DMN studies, was confirmed by structural MRI imaging and at times by the data of neurosurgical interventions. In what follows, we consider the results of our phenomenological analysis for the LIPC and RIPC groups, in that order.
Results
Phenomenology of the left hemisphere lesions
The central phenomenon in the behavior of LIPC patients was observed in a wide range of situations. We call it the “difficulty of entrance in the task” (DET), for the sake of simplicity. For example, during rehabilitation-exercises, a typical observation was that patients − after attentively following instruction to an exercise − started to work on it so poorly that this lead to an impression of their total inability to solve such category of tasks. However, after some delay filled with detailed explanation of the task by the neuropsychologist, patients suddenly arrived at the solution fast and correctly, leaving open the possible reason for their initial problems. The DET phenomenon is quite different to the dysexecutive (“frontal-lobe”) strategy of behavior, which is defined by impulsivity in decision making, as well as instability of attention and motivation. Here, in contrast, the level of achievement motivation was constantly elevated with often rather good scores in attention and immediate memory tests. Obviously, the difficulty was somehow related to understanding of the instruction and interpretation of the task situation by patients.
The same DET pattern of behavior was seen practically in all neuropsychological probes. We observed it even in very easy tasks that are typically clear from the scratch, without any additional explanations. For instance, in the Seguin Form Board test, where one has to place simple wooden forms into corresponding holes of the board, there usually is no need of instruction. Most subjects proceed to the correct solution in response to simply an inviting gesture and an encouraging head nod. This was not the case with LIPC patients. They started to explore forms, to sort and lay them out in a row etc., performing manipulations in no way related to the obvious solution. At the same time, there was no problem, in principle, in reaching the solution: shapes of the blocks were perfectly recognized and no difficulties in eye to hand coordination was observed. The problem was in the interpretation of the task situation. After a successful solution, if patients were asked for the reason of their initial reluctances, a typical answer was that at the beginning his/her first impression was that one had to find something complex and previously unknown which could not be immediately clear from the situation. Therefore, a straightforward solution was ignored.
To our knowledge, the DET phenomenon has never been stated in such a form in the past though there were many reports on categorization problems in patients with damages of left temporal lobe and temporo-parieto-occipital junction (Gelb, & Goldstein, 1920; Koivisto, & Laine, 2000; Wilkins, & Moscovich, 1978). As a matter of fact, a typical neuropsychological investigation focuses on analysis of task solution per se, i.e. the analysis starts only after the instruction is completely understood by the subject. This is possibly why the DET phenomenon, which is in essence the unusual interpretation of task situation, remained without due attention.
To model DET phenomenon in a common neuropsychological context, we sampled contrasting groups of patients with lesions in LIPC (n = 9) and RIPC (n = 8). Participants were given the Wisconsin Card Sorting Test (WCST). Test instruction is open to many interpretations: “I cannot explain to you how to solve this task. Please take cards one by one from the pile and place them on the four keycards. I will tell you every time whether you did it correct or incorrect”. On the cards there are figures which differ in shape, color and number. Subjects lay down cards by trial and error receiving experimenter’s evaluation as “correct” or “incorrect”. The category is considered to be learned if there are 10 errorless trials in a row. After that, the experimenter normally changes the categorization rule and the whole procedure repeats until the next 10 correct trials occur. In our study, we concentrated our analysis on the very first classifications registering the number of sortings needed to confirm understanding of the task instruction.
This reduced version of WCST revealed dramatic differences in performance of both patients’ groups. The RIPC group members needed only 2.1 trials on average to figure out the principle of the task and come up with a definite strategy of solution, whereas the LIPC patients needed as much as 12.2 trials to arrive at this understanding. These differences were highly significant (Wilcoxon-Mann-Whitney rank sum test, U = 9, p < 0.01). In should be stressed that following test classifications, when the first 10 errorless trials were achieved, differences in performance of both groups became non-significant. Similarly, no significant differences were observed between LIPC and RIPC patients in additional tests of working memory, selective attention and task switching. In fact, they often demonstrated better scores than “right-sided” patients. As to aphasic disturbances in LIPC patients, those were not serious enough to prohibit a dialog with experimenter. Thus, one can conclude that the DET phenomenon was successfully modeled in the experiment: our LIPC and not RIPC patients demonstrated selective problems in the initial phase of task situation by ignoring the simple and easy available solution. Of particular interest are self-reports of LIPC patients explaining their difficulties in finding a solution. Let us take color as the critical category. Typically, subjects with intact brains may make one or two wrong selections (shape or/and number) but thereafter come to the correct guess and solve the task. In LIPC patients, this expected sequence of events was never observed. One patient (female, 27 years, higher education, lesion resulted from a gunshot, with an alien object in the posterior parietal parts of left hemisphere), could not reach the solution after 25 trials. This happened despite her efforts to work with high accuracy. We interrupted the session in view of patient’s strain and negative emotional reactions to “incorrect” remarks. When asked to name features of cards, she mentioned shape, number, structure, spatial configuration but not color. Then we asked “And what about color?” – “Yes, color too, here is red, there are green, blue and yellow”. – “Why did you never attempt to sort cards by color?” ─ “I thought this would be too easy for a solution and that you gave me a more complex task”. Even if other members of the same group came up with color as the relevant category, they continued treating the task as being more complex than it in fact was. In three such cases we interrupted the test after 20 trials without solution. Afterward, these patients said that the emphasis on color seemed to be insufficient for them, so they looked for some “sequence algorithm” in the sorting. We also observed rather unusual hypothesis, when, for example, one patient decided to match cards to key samples in such a way that “no features will be in common”. Of course, such an excessively reflective strategy could not be successful in the WCST. All attempts at solution were done with maximum of efforts; sometimes one could see tears in patients’ eyes after next unsuccessful trial.
Thus, neither an attention deficit, nor memory weakness, nor lack of achievement motivation can directly explain this specific difficulty we detected on the early stage of task solution in patients with damage in left posterior cortical areas. Their problem lies in a general attitude towards task situations as a priori unique, i.e. requiring tough mental efforts and sophisticated strategies of problem solving. In the case of neuropsychological tests as well as everyday task situations, which all have low or middle levels of complexity, such a mode of thinking leads to overseeing obvious ways towards solution. “Permanent misunderstandings” were reported by relatives of these patients. We also observed these particularities during reha-exercises when simple movements could present LIPC patients with insurmountable strain, which led them to be suddenly “frozen” in an astonishment posture. The only way of overcoming such episodes was to start the explanation anew in an explicit top-down fashion: “We will now learn to walk properly, for this we have to perform several easy to learn exercises... To start with please repeat the movement, which I am showing you now”. There were large individual differences in the revealed picture of the “left-sided” mode of thinking which was observed on background of aphasic and motor disturbances to a different degree typical for patients. However, the central phenomenon of overcomplicating any task situation, as if it would need particular cognitive efforts, was present in all LIPC patients.
Phenomenology of the right hemisphere lesions
A completely different set of difficulties was observed in our RIPC patients. Before their description, we wish overview some peculiar features in behavior of these patients known from the literature and confirmed by our observations (see, e.g., Balonov, Deglin, & Tschernigovskaja, 1985; Kolb, & Whishaw, 2015; Luria, 1966). Right-handed RIPC patients do not usually have aphasic disturbances: their speech remains intact both grammatically and lexically. However, one often finds deficits in speech intonation structure. The patient’s voice loses its normal modulation of volume and cannot be voluntary regulated in a socially appropriate manner: it is either too quiet or loud and crude. Prosody of speech is also distorted – it becomes voiceless, husky, nasal or barking and shrill. Spontaneous speech makes a strange impression as monotonous and having no emotional expression. This cannot be changed even after an explicit instruction by the neuropsychologist, so special exercises are needed to correct the deficit. As a rule, this poverty of expression co-exists with similar deficits on the side of speech perception seriously complicating interpersonal communication (Koelsch, Kasper, Sammler, Schulze, Gunter, & Friederici, 2004). For instance, if one reads to the patient the same phrase with three intonations, that of doubt, mockery or fright, he/she will be unable to distinguish the variants by hearing.
Most of emotional expressivity is also lost in facial expression and gesture. The face loses its liveliness and gaze seems to be “frozen”. Often it is difficult for patients to recognize themselves in old photos “before” the trauma, as if it were other persons. Facial reactions do not disappear completely but e.g., smile has an unnatural, torturous character or becomes a coloration of euphoric comfort and some stupidity. It should be noted that patients with damage of left hemisphere demonstrate an opposite pattern of communicative abilities. Even with strong language disturbances like the aphasia of Wernike type, when patients have no single correct word in their lexicon so speech is a “word salad”, we can understand almost everything that they wish to say or to ask, can feel their mood and often maintain a rather informative dialog thanks to their intact facial expression, gesture and intonation.
With respect to problem solving behavior, RIPC patients do not demonstrate the slightest signs of DEP phenomenon. They easily manage the WCST and the variety of neuropsychological tests “on thinking”. However, a seemingly simple task from another domain suddenly is difficult for RIPC patients. For example, when we present them a picture depicting several personages who are in definite social relations to each other and the task is to explain these relations by thinking out loud one or two phrases for each personage, i.e. what everyone of the personages could say in this situation. Un our practice, we use for such tasks from “Stories in pictures” by N. Radlov (Radlov, Harms, Dilatorskaya, & Gernet, 2015/1937). This is a comic book for children in senior kindergarten. Though the pictures are without captions, depicted situations are so clear that children delightfully invent dialogs as all narratives have some humorous gist.
This sort of tasks led to substantial difficulties in our RIPC patients with lesions. They were able to –in general terms– describe the situation but could not reconstruct moods, intentions and possible remarks of depicted personages. As a rule, the concealed meaning, the very humorous spirit of every story in pictures was not discovered. Here is one example. In a series of three pictures, a man is depicted walking in a park with two puppies. When the wind takes his hat off, he orders puppies, by an imperious gesture, to bring it back. They do but tear the hat apart straggling for the master’s favor. A third picture shows the man whose posture and facial expression testify to his distress and confusion. After a long examination of pictures, one of the patients described the story in the following way: “A man walked with dogs. He threw them two hats that they brought back. The brown dog returned first and was praised by the man”. Here is another example which includes two pictures. On the first, there is a boy who undresses to go swim in a river and, without looking, he puts his hat on the corn of a cow standing in the bush behind him. The second picture shows the cow walking with the hat on the head, as well as the boy and a calf, both observing this with extreme astonishment. The patient’s story was as plain as following: “The boy hung his hat on the cow’s horn and she went away”. There were no appropriate descriptions of internal states, emotional exclamations or hypotheses about social interactions in the situation. Similar problems were detected with all forms of representations, including familiar photos and paintings. Being confronted with a reproduction of N. Ge’s “Peter I interrogating the Tsarevich Alexei in Peterhof” another patient correctly recognized the painting and remembered some events preceding this scene. He also easily described inanimate details of the interior. However being asked about feelings of persons in the scene, he replied in an overtly inadequate manner “Peter is in a good mood, he is joyful. Alexei feels conceit”.
Deficiencies in understanding emotional aspects of observed interpersonal communication coincided in RIPC patients with deficits of self-consciousness extended to their-own affective and mental states. Being presented with a set of photos showing people with different emotional expressions, they cannot find which one reflected their feelings and mood at that moment. For instant, a patient, in a state of intense irritation and just after two aggressive attacks against his mother sitting nearby, selected as a descriptor of his state the photo of a boy whose smiling face almost “eradiated” happiness. After a long search for an appropriate photo, another patient said: “No, there is no such photo here as I do not feel anything”. What we have seen as a dominant mood in these patients is a neutral placidity; sometimes it is interrupted by bursts of irritated aggression but there are no episodes of worries, fear or vivid happiness. Along with emotions, they seemed not to express common states such as fatigue. One of them, while obviously exhausted, negatively responded to our question about possible fatigue. Still another patient formulated his sensations in the following way: “I always cannot understand what it means “to be fatigued”. Should I have some pains? But I have no pains, nowhere”.
Besides these specific problems of self-referential cognition, we observed weakening of voluntary control of behaviors and meta-cognition in our RIPC patients. We already noted their difficulties with voluntary regulation of voice and emotional control. But this weakening had a more global character and is not necessarily focused on the emotional sphere. A patient could easily follow instruction “Close your eyes” but if you asked him to do something else, e.g., to grope about an object, the eyes reopened involuntary. A reminder that eyes should be closed did not work though patient was perfectly inclined to follow it: as soon as attention switched to another task, the eyes reopened to the astonishment of the patient. The same pattern applies to “asocial movements”, observed during patients’ involvement in complex tasks. A patient with a high premorbid status, adequate in social life and successful in almost all tests of the neuropsychological examination, could suddenly start scratching his body, digging into his nose or picking on something in his hairs, when a task demanded his full attention. After a remark from the experimenter that this is inappropriate in social situation, he got confused for a while but reappeared the behaviors if the task solution was effortful enough.
As to meta-cognition, i.e. deliberate regulation of own mental processes, it is weakened to the same substantial degree as control over external behaviors in RIPC patients. If we have nothing to do for some period of time, we are normally engaged in experiencing a kind of William James’ stream of consciousness which is in part under our control because it is always possible to turn its direction either to planning for the future or to remembering images from the past vacation. Our RIPC patients claimed that when they, for example, waited outside the room to begin their reha-exercises they had no thoughts. In order to launch thinking process, they seemed to need an external stimulus such as the advice of another person2. There were no complains about the lack of thoughts. Apparently, the patients did not experience boredom and had no intention to entertain them-selves in any way.
The emptiness of mental life was extended to self-referential aspects of remembering. Though patients’ performance in ordinary memory tests could be nearly perfect, we learned about salient autobiographical events of their lives almost exclusively from relatives because, as a rule, patients were unable to remember such subjectively colored information. Even photos of an event were of little help: only formal knowledge such as names of participating persons and general circumstances was retrieved but nothing that was mediated by subjective experience, either his/her-own (first-person perspective) or participants (second-person perspective) represented and correctly recognized in the photo. By borrowing terminology from A.N. Leontiev’s activity theory, only meaning was remembered not personal sense.
As a whole, these phenomena build a coherent picture which testifies to disrupted comprehension of interpersonal relationships in our RIPC patients. It clearly dissociates with their relatively intact formal knowledge and cognitive operations on information about inanimate objects, i.e. the domain of thinking in which it is possible to ignore subjectivity: feelings, beliefs and intentions. For the patients and their relatives this is a serious factor of invalidation in everyday situations. Errors with pragmatic context of communication, misunderstanding of other people’s emotions and inability to empathy destroy their social life: the mutual understanding with relatives disappears, friends start avoiding contacts, and overall alienation grows. In direct communication with others, they demonstrate symptoms of autism spectrum disorder, also with respect to their aberrant eye movement behavior eluding visual fixations on face and in particular on eyes of their vis-à-vis. Not less complicated is any form of indirect communication. For example, speaking to such a patient on the telephone, you never know for sure whether he/she is interested in a conversation or will hang up the next moment.
Discussion
We describe two qualitatively different patterns in everyday behavior and test performance in patients with damage in either left or right tertiary areas in the posterior cortex. Although there are large individual differences and interfering influences of other deficits (e.g., speaking and movement control in the LIPC group), the two patterns are very distinct. LIPC patients tend to overestimate the actual complexity of their surrounding world of things and standard social situations (such as in neuropsychological testing), whereas RIPC patients simplify complex social interactions by failing to attribute mental states to other people and experience these states by them-selves suggestive of deficits in self-referential and interpersonal cognition3 There are practical and theoretical consequences of these distinct characteristics. From the practical point of view, our results advocate for differential procedures in neurorehabilitation of patients with LIPC and RIPC damage. As even these procedures are not theory-free, a theoretical explanation of the emerging picture of a cognitive-affective architecture is the task of foremost importance, both for conceptual development and for practical applications. It should be said that the discovered pattern of hemispheric asymmetries questions the validity of traditional dichotomies and demands for a re-engineering of existing approaches.
The first of these dichotomies considers the left hemisphere as “leading” (or “dominant”, with respect to performance in simple sensorimotor probes in response to verbal instruction). This old dichotomy evidently reverses the real interactions and relative importance of lateralized brain mechanisms. In fact, damage of right hemisphere has a more serious deteriorating effect on everyday activities and social life of patients. This, in a sense, testifies to the leading role of the right hemisphere in realization of specifically human daily tasks. Another classical distinction of hemispheric asymmetry is that of “left is verbal and right is nonverbal”, which does not fare much better. Firstly, it is nonspecific, particularly with respect to the role of right hemisphere. Secondly, it ignores involvement of right hemisphere mechanisms in different forms of verbal processes (Federmeier, Mai, & Kutas, 2005; Winner, & Gardner, 1977), as well as the considerable role of left hemisphere in spatial operations of mental rotation, translation and zooming of imagined objects (Cohen et al., 1996; Mehta, & Newcombe, 1991). Thirdly, this distinction does not correspond to the rather not trivial picture of deficits revealed by the present study.
A standard model of hemispheric asymmetry popular in the last two decades explains it in terms of differential functioning in semantic memory (Atchley, Story, & Buchanan, 2001; Burgess, & Lund, 1998; Chiarello, 1998). Accordingly, the left hemisphere supports retrieval of high frequency associations, whereas the right hemisphere supports activates semantic relations with low frequencies of previous use. The model has been mainly used in neurolinguistics for explaining data on the hemispheric asymmetry in understanding of metaphoric language and indirect speech acts (Balonov, Deglin, & Tschernigovskaja, 1985; Shammi, & Stuss, 1999; Winner, & Gardner, 1977). Even in this narrow domain, the view does not receive support in more recent neuroimaging (Forgács et al., 2012) and divided visual field (Forgács, Lukács, & Pléh, 2014) experiments. The main problem with this hypothesis is that the observed phenomenology of hemispheric differences is richer than one implied by the frequency of semantic associations. Moreover, the theoretically implied direction of difference is opposite to one observed in reality. For example, the model cannot explain why damage of right hemisphere could lead to selective deficits in self-referential cognition and theory of mind, i.e. patients’ knowledge about knowledge, emotions and intentions of other people. Obviously, thoughts about emotions and intentions of one-self and others are especially frequent in the mental life of a typical healthy person.
In experiments, cognitive tasks, from perception of form to encoding information in terms of personal sense, show different patterns of asymmetries. For example, evaluation of some material as belonging to a certain semantic category leads to primary activation of left hemisphere, whereas encoding the same material in terms of its personal sense for the subject mostly activates right prefrontal cortex (Velichkovsky, Klemm, Dettmar, & Volke, 1996). In addition, hemispheric differences seem to have a long evolutionary history (Karenina, Giljov, Ingram, Rowntree, & Malashichev, 2017; Ocklenburg, & Güntürkün, 2012), therefore a broader approach describing several evolutionary steps, or levels in cognitive-affective organization need to be considered. Presuming that there is such a ‘‘vertical dimension’’ of mental functioning, what could granularity and distinct characteristics of levels be? It is clear that dichotomies are too unspecific. In the same vein, disagreement between authors of three-level theories implies that more levels may be at work4. The founder of biomechanics, N.A. Bernstein (1947), described four levels, from A to D, involved in realization of human movements. One of us upgraded his views some time ago, which led to a Grand design model with as many as six different levels of organization (Velichkovsky, 1990). The first group (from A to D) is primarily built up by the sensorimotor mechanisms. The second group (from E to F) consists of mechanisms of higher symbolic coordination. Here is the list of these levels in bottom-up order in a version which is about 15 years old (Velichkovsky, 2002, pp. 406-407).
Level A: Paleokinetic Regulations. Bernstein also called it the ‘‘rubro-spinal’’ level, having in mind the structures of spinal cord and brain stem (up to midbrain) involved in regulation of the muscles’ tonus as well as paleovestibular and basic defensive reflexes. The awareness of functioning is reduced here protopathic sensitivity (Head, 1920), which is so hedonistic, diffuse, and lacking any precise spatial coordinates (any definite “local signs”) that even the term sensation seems to be too intellectual in this case.
Level B: Synergies. Due to evolution of new neurological mechanisms—the ‘‘thalamo-pallidar system’’ after Bernstein—the broad sensory integration and regulation of the organism’s movements as a whole become possible, transforming it into a ‘‘locomotory machine’’. The specializations of this level are movements involving large groups of muscles of different body parts, e.g., rhythmic and cyclic patterns of motion underlying all forms of locomotion. Possibilities of awareness are limited to proprio- and tangoreceptoric sensations within the body’s frame of reference.
Level C: Spatial Field. The next round of evolution adds exteroception to the repertoire of sensory corrections. This opens outer 3d space and makes possible one-time goal/place-directed movements as well as topographically contingent behavior in the near environment. The control instances of the level are phylogenetically new parts of basal ganglia (striatum) and stimulotopically organized cortical areas, especially in posterior parietal cortex. The corresponding subjective experience is that of a stable voluminous surrounding filled with localized but only globally sketched objects.
Level D: Object Actions. A new spiral of evolution leads to the building of a variety of secondary areas of neocortex with parietal, premotor, and partially temporal regions as the main instances. This permits detailed form perception and object-adjusted manipulations. Individualized objects affording some but not other actions come to the focus of attention. Formation and tuning of sophisticated higher-order sensorimotor and perceptual skills is supported by a memory of the procedural type. Phenomenal experience is the perceptual image (as described by Gestalt school—e.g., Koffka, 1935).
Level E: Conceptual Structures. Supramodal associative cortices of temporo-parietal and frontal structures, particularly on the left side, provide the highest integration of various modalities supporting the ability to categorize objects and events as members of generic classes. Development of language and culture fosters this ability and virtually leads to formation of the powerful declarative-procedural mechanisms for symbolic representation of knowledge (widely but not quite correctly known as semantic memory). Common consciousness is the awareness mode at this level.
Level F: Metacognitive Coordinations. Changes in conceptual structures result not only from accretion of factual experience but also from experimentation with ontological (truth-value) parameters of knowledge. This ‘‘personal view of the world’’ and its counterpart, ‘‘theory of mind’’, are supported by those parts of the neocortex that show largest growth in anthropogenesis, notably by the prefrontal, especially, right prefrontal regions. This level provides resources for dealing with novel situations and tasks without (known) solution. It is behind self-referential and interpersonal processing, reflective consciousness, and productive imagination.
In the last decade, most of our experimental efforts aimed at refinement of the Grand design approach have been focused on two middle levels, C and D. These levels were related to both major pathways in development of sensory systems, dorsal and ventral “streams” (Velichkovsky, 2007; Velichkovsky, Joos, Helmert, & Pannasch, 2005). Seminal research on the role of hippocampus in episodic memory and in representation of surrounding space (Dickerson, & Eichenbaum, 2010; Moser, & Moser, 2008) opened the way to understanding of respective integration mechanisms in paleocortex whereby, in primates, dorsal stream information (“Where?”) propagates via parahippocampal structures and medial entorhinal area while ventral stream information (“What?”) seems to access the hippocampal formation through entorhinal cortex and lateral entorhinal area. Our recent data on effective connectivity of both hippocampi (Ushakov et al., 2016) show that a holistic multimodal representation of the surrounding space can be achieved only by the right hippocampus (see RHIP in Fig.1). This new result suggests a leading role of the right hemisphere with respect to primarily tasks of Level C. As to the present study, its main contribution is in correcting previous views ascribing metacognitive functions solely to prefrontal regions. Clearly, damages to the region including inferior parietal lobe and temporoparietal junction, namely RIPC, result in the kind of disturbances which could be expected after removal of mechanisms responsible for self-referential and interpersonal processing (Level F, of the Grand Design model).
Let us illustrate this by means of a scheme in Fig.2, where left and right sides signify structures of the left and right hemispheres. A removal of the upper box on the right side (i.e. the dark box with “F” on it) would lead to consequences which are simultaneously dramatic and very simple: the system loses its highest level of organization and as a result demonstrates the whole spectrum of disorders in reflective control and voluntary regulation of behavior. These negative changes are especially salient in the case of interpersonal relations and self-consciousness. Despite relatively preserved language mechanisms and intact basic cultural knowledge, patients with lesions of right hemisphere to a great extent lose their abilities for social communication. Moreover, together with emotional experience most of their personality vanishes as well. A completely different pattern of disturbances would arise after a removal of upper structure in the left part of Fig.2 (the dark box with “E”). This would lead to an unusual misbalance of the system’s architecture. The outfall of Level E (Conceptual Structures) with survival of Level F (Metacognitive Coordinations) would result in a paradox tendency of interpreting every task situation, even a trivial one, as a new challenge demanding some creative efforts. One can easily recognize the DET phenomenon in such surplus of redundant and often destructive contemplations. This phenomenon was systematically observed in problem solving behavior of our LIPC patients.
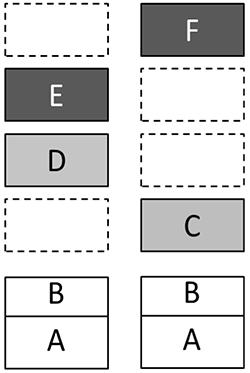
Figure 2. Grand Design model with the presumed asymmetry of multievel mechanisms in left and right hemispheres. Empty boxes mean that some levels are, at least, underrepresented on the side. (See text for explanation)
Thus, we demonstrated how differences in thinking of patients with disturbances of left and right hemispheres could be explained within the unified Grand Design framework. Of importance is however a more detailed understanding of relations between the levels with respect to their brain mechanisms. Large-scale brain mechanisms are most of the time in a state of dynamic balance. Any negative change in the architecture leads to a number of transformations, sometimes even to an exaggerated growth in other domains as it is the case with language development of children with Williams syndrome who otherwise have substantial deficits in spatial perception and thinking (Bellugi, Lichtenberger, Jones, Lai, & St. George, 2000). In our discussion of LIPC and RIPC patients, we emphasized symptoms characteristic for their specific modes of thinking after outfall of higher symbolic coordinations, but they also have a number of disorders in other neurological domains. In Fig. 2, main loci of the concomitant problems are marked with gray color. Damages to tertiary regions of the left hemisphere are frequently accompanied by pareses and dyspraxia as well as by disorders of object perception and reading. Within the Grand Design framework, these problems can be localized on the Level D.
A particularly variegated set of deficiencies can be observed in RIPC patients. Up to one third of them demonstrate neglect phenomena in one form or another. Most often this is the classical left-sided spatial hemi-neglect (Howard, & Templeton, 1966; Luria, 1966), which is a marked disorder of spatial field mechanisms, or Level C in the Grand Design model. But there are also distortions of corporeal awareness such as out-of-body experience (Blanke, & Mohr, 2005), asomatognosia (Baier, & Karnath, 2008) and anosognosia (Heilman, 2014), which are more difficult to attribute to spatial perception. In fact, this combination of symptoms ranging from distortions of bodily Self (“Koerper Ich” of old German authors) to that of higher-order thought, social intelligence and emotional processes is one of the greatest riddles in neuropsychology and cognitive science. Notions such as ‘embodied cognition’ are of not much help as they only rename the problem in unspecific terms.
Our working hypothesis is this. The role of explanans has the newly discovered right-ward lateralization of spatial representation abilities in the human parahippocampal regions (Ushakov et al., 2016). As we emphasized it above, cause-and-effect connections of the left –in contrast to the right-- hippocampal formation do not allow for a holistic representation of the surrounding space. If RIPC, its input to RHIP or perhaps RHIP itself are damaged, then all the tasks demanding a kind of personal appraisal may become problematical as an access to self-related cognitive-affective data cannot be easily found. Indeed, where could data related to “Self” be most easily found in the brain? As brain mechanisms have neither time not abilities to consult philosophical dissertations, it should be a simple heuristic. The simplest one is to search for self-related data at the obvious “Self” location, i.e. at the center of egocentric spatial representation. In normal conditions, it is the right hippocampal formation which provides the easy-to-find gateway into much of what we used to call “our subjective experience”. However, after right-hemisphere lesions, representation of “Self” may disappear or be somewhere shifted and lost within the scrambled spatial frame of reference leading to a variety of salient consequences for the behavior and mental life of RIPC patients.
Thus, clinical observations complemented by the methods of neurovisualzation and spectral DCM opened the way to the current progress in understanding of self-referential cognition and, potentially, its integration with emotional experience. Indeed, little attention was devoted in the discussion to the emotional life of our patients. Our data did not support the hypothesis about division of labor between hemispheres based on the emotional valence. If the mood of our LIPC patients had a reduced emotional flare this does not mean that mechanisms of positive emotions were somehow expressed by the left hemisphere but rather that these patients were able to realistic evaluation of their-own health condition. Conversely, in RIPC patients, the dominant mood was that of neutral placidity and mild euphoria, whereas no episodes of worries, fear or happiness were observed. Their deficit of self-referential and interpersonal processing explains this profile without reference to the alleged rightwards lateralization of negative emotions.
Conclusion
Patients with unilateral brain damage, either left or right, localized in posterior tertiary areas of the cortex (inferior parietal lobe and temporoparietal junction) present distinct patterns in everyday behavior, social competencies and problem solving. A systematic overestimation of task complexity even if the task was a trivial one was the main syndrome of the LIPC group. This overestimation resulted in a prolonged phase of redundant and often disruptive contemplations preceding task solution. A completely different pattern of difficulties was found in RIPC patients. Albeit their language and basic cultural skills were relatively preserved, they demonstrated serious disorders in experiencing emotions, theory of mind, metacognition and voluntary regulation of behavior. This pattern of results can be interpreted within a revisited multilevel framework (Velichkovsky, 1990; 2002). The revision concerns the fact that metacognitive functions usually ascribed to prefrontal regions are obviously related to posterior tertiary areas of right hemisphere as well.
The role of RIPC in personal appraisal can be furthermore explained by the strong asymmetry in causal connections of left and right hippocampi (Ushakov et al., 2016). Accordingly, an access to self-related data is based on the following heuristic: look for “ego”-related data at the center of egocentric spatial representation. Only the right hippocampus can provide such an easy-to-find gateway into what we call “subjective experience”. After right-hemisphere lesions, “Self” location within the bisected spatial frame of reference may be somewhere shifted and lost preventing access to and processing of self-related information. In a sense, such an exceptional function of the right hippocampus in the self-referential processes reminds one that was once attributed to the pineal gland on the reason that it is not an anatomically duplicated part of the brain and, thus, could serve as the site of the Aristotelian sensus communis. In the first formulation of this theory, Descartes wrote: ”And since it is the only solid part of the whole brain which is unique, it is necessary that it is the seat of the sensus communis, that is to say, that of thought, and as a consequence that of the soul; for the one cannot be separated from the other” (Descartes, 1640). In a similar vein, we can say that the right hippocampus is unique in its holistic representation of surrounding space, which seems to function as the common interface for the bodily “Self “ as well as for the higher-order thought and feelings.
Expanding the present focus of research to interconnected brain regions, such as the amygdala and prefrontal cortex would be consistent with the view that episodic memory performance depends on a synchronization of activities in the hippocampus and its brain’s environment (e.g. Fell et al., 2001). The latter includes temporal and frontal cortices with the amygdala as the major “amplifie” (McEwen, Nasca, & Gray, 2016). Recently, a priority of the right amygdala in functional connections with the ventrolateral prefrontal cortex has been reported (Kerestes, Chase, Phillips, Ladouceur, & Eickhoff, 2017). This can be a sign of more profound differences in the cause-and-effect connectivity of temporal and prefrontal cortices with the amygdalo-hippocampal region and underlying structures involved in regulation of basic needs and emotional reward of activity. In any case, the current knowledge about contrasting functions of posterior tertiary areas of left and right hemisphere will be an essential component of modeling human cognitive-affective architecture in the years to come.
Acknowledgements
This study was in part supported by the Russian Science Foundation (grants 14-28-00234 and 17-78-30029 aimed at investigation of self-referential cognition and semantic representations in the human brain, respectively) and by the Russian Foundation for Fundamental Research (ofi-m grants 15-29-01344 and 17-29-02518 as related to attentional control and to the large-scale network organization, respectively). We wish to thank Marie Arsalidou and Olga Efimova for their help in formulating our ideas about brain mechanisms of consciousness.
References
- Arsalidou, M., Pascual‐Leone, J., Johnson, J., Morris, D., & Taylor, M. J. (2013). A balancing act of the brain: Activations and deactivations driven by cognitive load. Brain and Behavior, 3(3), 273-285. doi: 10.1002/brb3.128
- Atchley, R.A., Story, J., & Buchanan, L. (2001). Exploring the contribution of the cerebral hemispheres to language comprehension deficits in adults with developmental language disorder. Brain and Cognition, 46, 16-20. doi: 10.1006/brcg.2000.1268
- Baier, B., & Karnath, H.O. (2008). Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 39(2), 486-488. doi: 10.1161/STROKEAHA.107.495606
- Balonov, L.Ja., Deglin, V.L., & Chernigovskaja, T.A. (1979). Funktionalnaja asimmetrija mozga v organizatii rechvoj aktivnosti [Functional brain asymmetry in organization of language activity]. In Sensornie sistemy [Sensory systems] (pp. 35-39). Leningrad: Nauka.
- Bellugi, U., Lichtenberger, L., Jones, W., Lai, Z., & St. George, M. (2000). The neurocognitive profile of Williams syndrome: A complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience, 12 (Suppl. 1), 7-29. doi: 10.1162/089892900561959
- Bernstein, N.A. (1990). O postrojenii dvizhenij [On the construction of movements]. In N.A. Bernstein, Physiologija aktivnosti [Physiology of activity]. Moscow: Nauka. (Original work published 1947)
- Bischof-Koehler, D. (1989). Spiegelbild und Empathie: Die Anfaenge der sozialen Kognition [Mirror image and empathy]. Bern: Huber.
- Blanke, O., & Mohr, C. (2005). Out-of-body experience, heautoscopy, and autoscopic hallucination of neurological origin: Implications for neurocognitive mechanisms of corporeal awareness and self-consciousness. Brain Research Reviews, 50(1), 184-199. doi: 10.1016/j.brainresrev.2005.05.008
- Buckner, R.L., Andrews-Hanna, J.R., & Schacter, D.L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1-38. doi: 10.1196/annals.1440.011
- Burgess, C., & Lund, K. (1998). Modeling cerebral asymmetries in high-dimensional space. In M. Beeman & C. Chiarello (Eds.) Right hemisphere language comprehension: Perspectives from cognitive neuroscience (pp. 215-244). Mahwah, NJ: Lawrence Erlbaum.
- Burgess, N., Jackson, A., Hartley, T., & O’Keefe, J. (2000). Predictions derived from modeling the hippocampal role in navigation. Biological Cybernetics, 83, 301-312. doi: 10.1007/s004220000172
- Burgess, P.W., Cohen-Yaacovi, G., & Volle, E. (2012). Rostral prefrontal cortex. In B. Levine & F.I.M. Craik (Eds.) Mind and the frontal lobes: Cognition, behavior, and brain imaging (pp. 47-92). New York: Oxford University Press.
- Carper, R.A., Treiber, J.M., DeJesus, S.Y., & Müller, R.-A, (2016). Reduced hemispheric aymmetry of white matter microstructure in Autism Spectrum Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55(12), 1073-1080. doi: 10.1016/j.jaac.2016.09.491
- Chiarello, C. (1998). On code of meaning and the meaning of codes: Semantic access and retrieval within and between hemispheres. In M. Beeman & C. Chiarello (Eds.) Right hemisphere language comprehension: Perspectives from cognitive neuroscience (pp. 141-160). Mahwah, NJ: Lawrence Erlbaum.
- Cohen, M.S., Kosslyn, S.M., Breiter, H.C., DiGirolamo, G.J., Thompson, W.L. Anderson, A.K., … Belliveau, J.W. (1996). Changes in cortical activity during mental rotation: A mapping study using functional magnetic resonance study. Brain, 119, 89-100. doi: 10.1093/brain/119.1.89
- Craik, F.I.M., Moroz, T.M., Moscovitch, M., Stuss, D.T., Winocur, G., Tulving, E., & Kapur, S. (1999). In search of the Self: A positron emission tomography study. Psychological Science, 10(1), 26-34. doi: 10.1111/1467-9280.00102
- Descartes, R. (1640). Letter to Mersenne, 24 December 1640. In: C. Adam & P. Tannery (Eds.) (1964-1974). Oeuvres de Descartes (vol. III, no. 223), 2nd ed., Paris: Vrin.
- Dickerson, B. C., & Eichenbaum, H. (2010). The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology Reviews, 35, 86-104. doi: 10.1038/npp.2009.126
- Dolina, I.A., Efimova, O.I., Kildyushov, E.M., Sokolov, A.S., Khaitovich, P.E., Nedoluzhko, A.V., . . . Velichkovsky, B.M. (2017). Exploring terra incognita of cognitive science: Differential gene expression in the most rostral parts of human brain. Psychology in Russia: State of the Art, 10(3), 231-247. doi: 10.11621/pir.2017.0316
- El-Gaby M., Shipton, O.A., & Paulsen, O. (2015). Synaptic plasticity and memory: New insights from hippocampal left-right asymmetries. Neuroscientist, 21(5), 490-502. doi: 10.1177/1073858414550658.
- Forgács, B., Bohrn, I., Baudewig, J., Hofmann, M.J., Pléh, C., & Jacobs. A.M. (2012). Neural correlates of combinatorial semantic processing of literal and figurative noun compound words. NeuroImage, 63, 1432–1442. doi: 10.1016/j.neuroimage.2012.07.029
- Forgács, B., Lukács, Á., & Pléh, C. (2014). Lateralized processing of novel metaphors: Disentangling figurativeness and novelty. Neuropsychologia, 56, 101-109. doi: 10.1016/j.neuropsychologia.2014.01.003
- Federmeier, K.D., Mai, H., & Kutas, M. (2005). Both sides get the point: Bihemispheric sensitivity to sentential constraint. Memory and Cognition, 33, 871-886. doi: 10.3758/BF03193082
- Gazzaniga, M.S., (Ed.). (2009). The cognitive neuroscience. 4th Ed. Boston, MA: MIT Press.
- Gelb, A., & Goldstein, K. (1920). Psychologische Analysen hirnpathologischer Faelle [Psychological analyses of brainpathology cases]. Leipzig: Barth.
- Gusnard, D.A., Akbudak, E., Shulman, G.L., & Raichle, M.E. (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. PNAS, 98, 4259–4264. doi: 10.1073/pnas.071043098
- Gusnard, D.A., & Raichle, M.E. (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience, 2, 685–694. doi: 10.1038/35094500.
- Head, H. (1920). Studies in neurology. Oxford: Oxford University Press.
- Heilman, K.M. (2014). Possible mechanisms of anosognosia of hemiplegia. Cortex, 61, 30-42. doi: 10.1016/j.cortex.2014.06.007
- Howard, I.P., & Templeton, W.B. (1966). Human spatial orientation. London: Wiley.
- Karenina, K., Giljov, A., Ingram, J., Rowntree, V. J. & Malashichev, Y. (2017). Lateralization of mother–infant interactions in a diverse range of mammal species. Nature Ecology & Evolution, 1, 0030. doi: 10.1038/s41559-016-0030
- Kerestes, R., Chase, H.W., Phillips, M.L., Ladouceur, C.D., & Eickhoff, S.B. (2017). Multimodal evaluation of the amygdala's functional connectivity. NeuroImage, 148, 219–229. doi: 10.1016/j.neuroimage.2016.12.023
- Khomskaja, E.D. (1987). Neuropsikhologia [Neuropsychology]. Moscow: Prosveshchenie.
- Koelsch, S., Kasper, E., Sammler, D., Schulze, K., Gunter, T.C. & Friederici, A.D. (2004). Music, language, and meaning: Brain signatures of semantic processing. Nature Neuroscience, 7, 302-307. doi: 10.1038/nn1197
- Koffka, K. (1935). Principles of Gestalt psychology. New York: Harcourt, Brace & World.
- Koivisto, M., & Laine, M. (2000). Hemispheric asymmetries in activation and integration of categorical information. Laterality, 5, 1-21. doi: 10.1080/713754358
- Kolb, B., & Whishaw, I.Q. (2015). Fundamentals of human neuropsychology. 7th Ed. New York: Worth Publishers.
- Korsakova, N.K., & Moskovichute, L.I. (1988). Klinicheskaja neuropsihologija [Clinical neuropsychology]. Moscow: Moscow University Press.
- Kortte, K.B. Wolfman McWhorter, J., Pawlak, M.A., Slentz, J., Sur, S., & Hillis, A.E. (2015). Anosognosia for hemiplegia: The contributory role of right inferior frontal gyrus. Neuropsychology, 29(3), 421–432. doi: 10.1037/neu0000135
- Krotkova, O.A., & Velichkovsky, B.M. (2008). Mezhpolusharnije razlichija myshlenija pri porazhenijah visshih gnosticheskih otdelov mozga [Hemispheric differences of thinking in the damages of higher gnostic brain regions]. In B.M. Velichkovsky & V.D. Soloviev (Eds.). Komputery, mozg, poznanie [Computers, brain, cognition] (pp. 107-132). Moscow: Nauka.
- Luria, A.R. (1966). Higher cortical function in man. NewYork: Basic Books.
- Luria, A.R., & Tsvetkova, L.S. (1966). Neuropsychologicheskij analis reshenija zadach [Neuropsychological analysis of problem solving]. Moscow: Moscow University Press.
- McEwen, B.S., Nasca, C., & Gray, J.D. (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology Reviews, 41(1), 3-23. doi: 10.1038/npp.2015.171
- Mehta, Z., & Newcombe, F. (1991). A role for the left hemisphere in spatial processing. Cortex, 27(2), 153-167. doi: 10.1016/S0010-9452(13)80121-9
- Moser, E.I., & Moser, M.B. (2008). A metric for space. Hippocampus, 18, 1142–1156. doi:10.1002/hipo.20483.
- Ocklenburg, S., & Güntürkün, O. (2012). Hemispheric asymmetries: The comparative view. Frontiers in Psychology, 3, 1-9. doi: 10.3389/fpsyg.2012.00005
- Penfield, W., & Evans, J. (1935). The frontal lobe in man: A clinical study of maximum removals. Brain, 58, 115-133. 10.1093/brain/58.1.115
- Radlov, N.E., Harms, D., Dilatorskaja, N.L., & Gernet, N. (2015). Rasskazy v kartinkakh [Stories in pictures]. Moskva: Melik-Pashaev Publishing House. (Original work published 1937)
- Raichle, M.E., MacLeod, A.M., Snyder,A.Z., Powers,W.J., Gusnard,D.A., & Shulman, G.L. (2001). A default mode of brain function. PNAS, 98, 676-682. doi: 10.1073/pnas.98.2.676
- Robinson, J.L., Salibi, N., & Deshpande, G. (2016). Functional connectivity of the left and right hippocampi: Evidence for functional lateralization along the long-axis using meta-analytic approaches and ultra-high field functional neuroimaging. Neuroimage, 27, 64-78. doi: 10.1016/j.neuroimage.2016.04.022
- Schilbach, L., Eickhoff, S.B., Rotarska-Jagiela, A., Fink, G.R., & Vogeley, K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cognition, 17, 457-67. doi: 10.1016/j.concog.2008.03.013
- Shammi, P., & Stuss, D.T. (1999). Humor appreciation:A role of the right frontal lobe. Brain, 122, 657–666. doi: 10.1093/brain/122.4.657
- Sharaev ,M., Zavyalova, V., Ushakov, V.L., Kartashov, S.I., & Velichkovsky, B.M. (2016). Effective connectivity within the default mode network: Dynamic causal modeling of resting-state fMRI data. Frontiers in Human Neurosciences, 10, 14. doi: 10.3389/fnhum.2016.00014
- Shipton, O.A., El-Gaby, M., Apergis-Schoute, J., Deisseroth, K., Bannerman, D.M., Paulsen, O., & Kohl, M.M. (2014). Left-right dissociation of hippocampal memory processes in mice. PNAS, 111(42), 15238-41523. doi: 10.1073/pnas.1405648111.
- Singh-Curry, V., & Husain, M. (2009). The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia, 47, 1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033
- Sokolov, E.N. (2013). Psychophysiology of consciousness. New York: Oxford University Press.
- Stuss, D.T., Rosenbaum, R.S., Malcom, S., Christiana, W. & Keenan, J.P. (2005). The frontal lobes and self-awareness. In T.E. Feinberg & J.P. Keenan (Eds.), The lost Self: Pathologies of the brain and identity (pp. 50-64). NY: Oxford University Press. doi: 10.1093/acprof:oso/9780195173413.003.0005
- Ushakov, V.L. Sharaev, M.G., Kartashov, S.I., Zavyalova, V.V., Verkhlyutov V.M., & Velichkovsky, B.M. (2016). Dynamic Causal Modeling of hippocampal links within the human default mode network: Lateralization and computational stability of effective connections. Frontiers in Human Neuroscience, 10, e64466. doi: 10.3389/fnhum.2016.00528
- Vallar, G., Bottini, G. & Sterzi, R. (2003). Anosognosia for left-sided motor and sensory deficits, motor neglect, and sensory hemiinattention: Is there a relationship? Progress in Brain Research, 142, 289-301. doi: 10.1016/S0079-6123(03)42020-7
- Velichkovsky, B.M. (1990). The vertical dimension of mental functioning. Psychological Research, 52, 282-289. doi: 10.1007/BF00877536
- Velichkovsky, B.M. (2002). Heterarchy of cognition: The depths and the highs of a framework for memory research. Memory, 10(5/6), 405-419. doi: 10.1080/09658210244000234
- Velichkovsky, B.M. (2007). Towards an evolutionary framework for human cognitive neuroscience. Theoretical Biology, 2(1), 3-8. doi: 10.1162/biot.2007.2.1.3
- Velichkovsky, B.M., Joos, M., Helmert, J.R., & Pannasch, S. (2005). Two visual systems and their eye movements: Evidence from static and dynamic scene perception. In B. G. Bara, L. Barsalou & M. Bucciarelli (Eds.), Proceedings of the XXVII Conference of the Cognitive Science Society (pp. 2283–2288). Mahwah, NJ: Lawrence Erlbaum.
- Velichkovsky, B.M., Klemm, T., Dettmar, P., & Volke, H.-J. (1996). Evozierte Kohaerenz des EEG II: Kommunikation der Hirnareale und Verarbeitungtiefe [Evoked coherence of EEG II: Communication of brain areas and depth of processing]. Zeitschrift fuer EEG-EMG, 27, 111–119.
- Vincent, D.J., Bloomer, C.J., Hinson, V.K., & Bergmann, K.J. (2006). The range of motor activation in the normal human cortex using bold FMRI. Brain Topography, 18, 273–280. doi: 10.1007/s10548-006-0005-y
- Wilkins, A., & Moscovich, M., (1978). Selective impairment of semantic memory after temporal lobectomy. Neuropsychologia, 16, 73-79. doi: 10.1016/0028-3932(78)90044-1
- Winner, E., & Gardner, H., (1977). The comprehension of metaphor in brain-damaged patients. Brain, 100, 717-729. doi: 10.1093/brain/100.4.717





