Understanding Mental Models of Dilution in Thai Students


Опубликована Апрель 10, 2009
Последнее обновление статьи Дек. 30, 2022
Abstract
The purpose of this study was to investigate Thai students’ understanding of dilution and related concepts. The literature suggests that a complete understanding of chemistry concepts such as dilution entails understanding of and the ability to integrate mental models across three levels of representation: the macroscopic, sub-microscopic and symbolic. In this work students’ understanding was probed using the interview about events (IAE) approach employing open-ended questions, and also by analysis of student descriptions, and drawings. The research findings suggest that all students were able to answer openended questions related to dilution and related concepts. Less able students presented representations at the symbolic level and subsequently described events at the submicroscopic and macroscopic levels. However, these latter representations typically were unrelated to the representations presented at the symbolic level. In contrast, more able students were able to present consistent representations of dilution at each level of representation.
Ключевые слова
Dilution, macroscopic level, symbolic level, mental model, sub-microscopic level
Introduction
Mental Models
Mental models represent ideas in an individual’s mind that they use to describe and explain phenomena. According to Van Der Veer and Del Carmen Huerta Melguizo (2003), mental models are constructed from perception, imagination, or from the comprehension of discourse. When studying science, students gain knowledge of scientific mental models as a result of exposure to the teaching of such models (Harrison & Treagust, 2000). That is, students create their own mental models when they learn and try to understand scientific knowledge during the learning process (Chittieborough, Treagust, Mamiala, & Mocerino, 2005).
In science “mental models are used to describe a system and its component parts as well as its states, to explain its behavior when changing from a state to another and to predict future states of the system” (Franco & Colinvaux, 2000, p. 105). According to Coll (1999), mental models are used to produce simpler forms of concepts, to provide stimulation and support for the visualization, and to provide explanations for scientific phenomena (Coll, 1999). The literature suggest that mental models play a central role in science (Gilbert, Boulter, & Rutherford, 2000; Gobert & Buckley, 2000) and in the communication of scientific knowledge (Dagher, 1994; Treagust, 1993). In science teaching, teachers use mental models in two distinct ways. First, they try to communicate the models of science (e.g., atomic structure) to their students. Second, they use certain types of models - particularly analogy, to explain scientific ideas to students (Duit, 1991). Students likewise construct their own individual mental models, and also to try use them to understand scientific phenomena that they encounter during instruction or in everyday life (Duit, 1991; Pittman, 1999; Venville, Bryer, & Treagust, 1994). However, the literature suggests that student understanding of science models, including mental models, and some of their own constructed mental models are often at variance with scientific models (Greca & Moreira, 2000; Norman, 1983).
Mental Models and Explanations of Experts and Novices
A science expert is a person who is recognized as a reliable source of knowledge, technique, or skill with significant experience gained through practice and education in a particular scientific field. In contrast, a novice is a person who is newly introduced to any science or another field of study and undergoing training in order to meet normal requirements of being regarded a mature and equal participant in a community of practice (e.g., science). Experts and novices are different in terms of four basic processes: knowledge, representation, problem-solving, and understanding (Heyworth, 1999; Savelsbergh, De Jong, & Ferguson-Hessler, 2002). Savels- bergh et al. (2002) investigated the differences between experts and novices in gaining knowledge: content, structure, and sources. It seems that the experts are able to modify and to construct representations, and also are highly capable at problem-solving due to a high qualitative content knowledge in their fields. In contrast, novices lack this qualitative content knowledge, and typically seek to solve problems by use of quantitative representations, and rote use of formulae. Kozma and Russell (1997) observe that experts use underlying principles to categorize their representations and transformed representations. In order to shift from novice to expert capability, it is necessary to enhance problem-solving capability. To do this we need to develop their declarative knowledge to help them to learn how to synthesize knowledge, to create the mental models, and to recognize similarities across many problems (Foshay & Kirkley, 2003).
Famham-Diggory (1994) identified five types of knowledges: declarative, procedural, conceptual, analogical and logical. Declarative knowledge is things that we know; it consists of the facts, concepts and principles for a knowledge domain. Famham-Diggory claim that knowledge is stored in an individual’s cognitive structures, and that a person constructs associations, lists, scripts, plans, schemata and mental models. Of these associations, mental models are deemed the most powerful cognitive structures, and work to relate procedural and declarative knowledge. Anderson (1995) describes mental models as the synthesis of declarative knowledge to solve problems. Jonassen and Tessmer (1996), however, believe that the mental models are distinct from declarative and procedural knowledge.
Grosslight, Unger, Jay, and Smith.(1991) report that mental models help experts to understand and think about phenomena and are used to formulate and test real ideas. They go on to note that a person’s understanding of mental models can be categorized into three levels. At level 1, models are seen as a ‘toy’ or a ‘copy’ of reality. At level 2, models serve a specific and explicit purpose. At level 3, models are constructed to develop and test an idea, and can be manipulated and subjected to test, final, Qalik, Ayas, and Coll (2006) similarly classify the level of student’s understanding into three categories: reasoning ability, hierarchy of qualitatively different understandings, and structural characteristics.
Mental Models and Levels of Representation
Chemistry is composed of many abstracts concept and topics (Gabel, 1999; Johnstone, 1993). When describing chemistry phenomena, chemists generally present concepts at three levels of knowledge representation: the macroscopic, sub-microscopic, and symbolic levels (Johnstone, 1991).
- The macroscopic level, is a concrete level corresponding to observable objects. At this level, students observe the chemical phenomena in their experiments or experiment (Johnstone, 1991; Treagust, Chittieborough, & Mamiala, 2003);
- The sub-microscopic level, is an abstract level, but corresponding to observable phenomena at the macroscopic level. This level is characterized by concepts, theories and principles used to explain what is observed at the macroscopic level, using things such as the movement of electrons, molecules, or atoms (Johnstone, 1991);
- The symbolic level, is used to represent chemical and macroscopic phenomena by the use of chemical equations, mathematical equations, graphs, reaction mechanisms, analogies and model kits (Johnstone, 1991).
Gabel (1999) says that chemical phenomena, which we observe or study at the macroscopic level, can also be described and explained at the sub-microscopic level. But are generally described at the sub-microscopic and symbolic levels in order to solve they complicated problems. Students are apparently able to understand complex ideas better when asked to express the relationships between macroscopic and sub-microscopic levels using chemical symbols, chemical equations and mathematical equations.
Johnstone (1993) says that in order to understand scientific knowledge, students must understand the knowledge at three levels of representations: macrochemistry, submicrochemistry and representational chemistry. In order to integrate the three levels of representation, students need to confront a variety of problems: fragmented topics that do not fit well rather than be presented with simple problems that have one, rather obvious answer (Gabel, 1999). Second, they need to learn how to connect abstract representations (Wu, Krajcik, & Soloway, 2001), and third they need to be exposed to abstract phenomena that are difficult to interpret or visualize at the sub-microscopic and symbolic levels (Johnstone, 1991).
Johnstone (1991) and Treagust et al. (2003) argue that the three levels of representation are interconnected in a triangle analogy as shown in Figure 1. The macroscopic level is the basis of chemistry, and teachers usually explain what happens at this level by using symbolic and sub-microscopic levels (Johnstone, 1991; Treagust et al., 2003).
Devetak (2005) developed the Interdependence of Three Levels of Science Concepts (ITLS) model in order to explain the connection between concrete and abstract levels as shown in Figure 2. In order to induct knowledge into their long-term memory, students should be encouraged to use mental models in order to see how to connect all three levels (Devetak, Vogrinc, & Glazar, in press).
If students understand the role of each level of chemical representation, they can often then see how transfer knowledge from one level to another - meaning they are able to generate understandable explanations, thus reducing alternative conceptions (Russell et al., 1997; Trea- gust et al., 2003). Gabel (1999) states that if students see how the three levels are interconnected then students are able to generate relational understanding - reducing alternative conceptions (Mulford & Robinson, 2002; Russell et al., 1997; Treagust et al., 2003).
Making transitions between the three levels of representations is apparently difficult for students. Hinton and Nakhleh (1999) report that chemical phenomena are generally understandable at the macroscopic level, and able to be interpreted at the symbolic level by undergraduate students. However, it seems students are often unable to connect either of these levels to the sub-microscopic level. Nurrenbem and Pickering (1987) likewise report that students do not understand the nature of matter, and they are unable to imagine the nature of particles. This means students also struggle to connect the symbolic level with the sub- microscopic level. Dori and Hameiri (2003) support this saying that students can see, touch, or smell things when doing experiments at the macroscopic level, but find it difficult to explain the nature of matter at the symbolic level.
Gabel (1993) states that students should be encouraged to understand the cross relationships in daily life. A variety of instructional approaches have been used to help students understand chemistry at the three levels of representation (Wu et al., 2001): instructional technology (Ardac & Akaygun, 2004; Russell et al., 1997; Tasker & Dalton, 2006), laboratory activities (Chandrasegaran, Treagust, & Mocerino, 2008; Gabel, 1999), mental models (Chit- tleborough & Treagust, 2007) and concrete models (Copolo & Hounshell, 1995). In addition, numerous researchers have used the three levels of representation in order to probe students’ deep understanding (Bowen, 1998; Raviolo, 2001). Interestingly, it seems that secondary school students who learn the chemical concepts at the three levels of representation are able to solve problems better than first year students (Devetak, Urbancic, Wissiak Grm, Kmel, & Glazar, 2004).
A particular issue arises when trying to teach students chemistry concepts involving representation at all three levels. Chemistry teachers often provide students with algorithms or formulas for solving chemical problems. It seems this mostly occurs because of pressure to ‘perform’ in external summative examination which reward correct numerical answers (Dah- sah & Coll, 2008). However, it seems students often use mathematical equations without understanding them in terms of the underlying chemistry or science concepts. In order to acquire the ‘right’ answers, they usually memorize the mathematical equations and plug in numbers, rather than attempt to solve problems using the basic concepts (Beall & Prescott, 1994; Bunce, Gabel, & Samuel, 1991; Lythcott, 1990; Robinson, 2003). Interestingly, Bunce et al. (1991) report that students are often able to ‘solve’ chemistry problems numerically. But this does not mean they actually understand the related chemistry. Dahsah and Coll (2008) say students are better able to solve new problems if they understand the basic chemistry concepts first.
Mental Models for Dilution
An understanding of dilution methods is an important part of much introductory chemistry, particularly in the laboratory. For example, most chemistry experiments require students to know how to prepare solutions of known concentrations (e.g., standard solutions) or to dilute solutions of known concentration (Dunnivant, Simon, & Willson, 2002; McElroy, 1996; Wang, 2000). Dahsah and Coll (2007) point out that when students cannot solve problems it is often because they misunderstand the related underlying concepts (e.g., solvent, solute, solution, concentration, solubility, and the mole). Additionally, there are other topics such as volume and molecules which are embedded in the above concepts, and which are needed to solve numerical problems. Dilution and related concepts are abstract and difficult leading to many student alternative conceptions such as the relationship between amount of solute and volume of solution (Dahsah & Coll, 2008; Devetak et al., in press) a diluted solution (Qalik, 2005), the relationship between solvent and solute (Qalik & Ayas, 2005; Devetak et al., in press) the solution concentration at particulate level (Devetak et al., in press) , and the meaning of homogenous solutions (Qalik & Ayas, 2005).
Hence, in summary, students’ understanding of dilution and related concepts is typically evaluated by consideration of their ability to solve numerical problems (Dahsah & Coll, 2007; Staver & Lumpe, 1995) but this does not necessarily mean they understand related chemistry concepts (Qalik, 2005; Case & Fraser, 1999; Dahsah & Coll, 2007; Pinarbasi & Canpolat, 2003; Schmidt & Jigneus, 2003).
Research Purpose
The purpose of this study was to investigate and evaluate Thai undergraduate students’ understanding of dilution and related concept by accessing their mental models. We also were interested to see how they were able to make connections between the macroscopic and sub- microscopic levels (Coll & Treagust, 2003a, 2003b). The research aims for this study were:
- To identify how students tried to explain dilution topics at the macroscopic, sub- microscopic, and symbolic levels; and
- To evaluate student understanding for dilution and related concepts.
Theoretical Basis to the Study
We suggest here that in order to answer the research aims, what we need to do is access the mental models students possess for dilution concepts. Harrison and Treagust (2000) say all representations of chemistry concepts are in fact expressions of students’ mental models. Hence, here we draw upon literatures about mental models to underpin the study. The theoretical basis of this study was based thus on Norman’s (1983) typology of mental models. According to Norman mental models can be classified into four types: the target system, the conceptual model of that target system, the user’s mental model of the target system, and the scientist’s conceptualization of the target system (Norman, 1983). Coll and Treagust (2003b) comment that the target model is the model we are striving to teach. Thus to link this to scientist’s conceptualization of the target system, necessitates a synthesis of mental models for the target system, situated into the particular context in which the teaching and learning occurs (i.e., in this case Thailand). This was achieved from a synthesis of curriculum materials; namely, textbook descriptions of concepts, analysis of lecture notes and lesson plans. During teaching students try to understand abstract concepts in chemistry and in the present work we tried to access the students’ metal model (i.e., the users’ mental model to employ Norman’s term) for the target system. In doing so (see below for the specific approach used) we requested participants to depict their models at three levels of chemical representations: macroscopic, sub-microscopic, and symbolic, and got them to try to relate each level (Russell et al., 1997; Treagust et al., 2003).
Methodology
Sample
The sample used in this study consisted on an entire intact class of 414 first-year undergraduate students (aged 18-19 years) who were enrolled in the SCCH 108 chemistry laboratory I in the at Mahidol University. The students consisted of male (39.10%) and female (60.90%). Mahidol University is at autonomous university that is one of the most prestigious universities in Thailand. It is located at Bangkok, Thailand.
Procedures
The first year class was divided into 23 groups, with each group supervised by a teaching assistant. Each group was then divided into 4-5 subgroups; and the sub-groups performed experiments about dilution and related concepts. The students were assigned to do hands-on activi
ties to evaluate their understanding of dilution and related concepts. They performed experiments and write-up a laboratory report in group. The purpose of this was to evaluate the students’ academic ability before the data collection phase (see below).
Data Collection
The researcher first analyzed scores from student laboratory reports. The results suggested that the students could be classified into two groups (A and B) based on an evaluation of their laboratory reports - as high or low ability.
- Group A: High ability students (students Al, A2, АЗ, A4, and A5) These students’ scored more than 70%.
- Group B: Low ability students (students Bl, B2, B3, B4, and B5) These students’ scored less than 70%.
Ten students volunteered to be interviewed by IAE technique and were subsequently interviewed using the interview-about-events technique (IAE) championed by Gilbert, Watts, and Osborne (2005) to probe the students’ mental models. Three approaches were used to investigate the students’ mental models at the same time - as is normal in the IAE technique: open-ended questions, drawing with description, and interview data. Students’ mental model was elicited their understandings at three levels of representation: macroscopic, sub- microscopic and symbolic. The interview protocol was developed from a pilot study in a similar manner to that reported in Coll and Treagust (2003a, 2003b). The IAE focus cards were developed based on the eight-step algorithm reported by Gilbert et al. (2005). In the interviews, the students were encouraged to speak freely, the cards were designed to connect the events to possible students’ life experiences (Ünal, Qalik, Ayas, & Coll, 2006).
There were three IAE focus cards employed and these were labeled DA 01, DA 02, and DA 03: DA 03 (see appendix). The interviewer used these IAE focus cards to conduct a 60- minute interview with four students for each of the achievement levels described above, and followed the protocol detailed below (i.e., open-ended questions, drawing with description, and interview questions to clarify meaning of drawings or responses).
Task 1 IAE Focus Card DA01
- Students were asked to explain what they understood about a 1 M solution of sodium chloride by giving an explanation in terms of solvent, solute, and homogeneity of solution.
- Students were also asked to depict a 1000 mL of 1 M NaCl at the macroscopic, sub-microscopic, and symbolic levels.
Task 2 IAE Focus Card DA02
- The interviewer showed participants a can of Coke with a label showing that it was a 10 %w/v sugar solution. Then students were asked to describe what they understood by this term.
- The interviewer showed participants IAE focus card DA02 and asked ‘Which figure shows the most diluted Coke beverage?’ Participants were asked to depict what they understood at the macroscopic, sub- microscopic, and symbolic level.
- Participants were asked to link IAE focus card DA02 with a solution and a diluted solution.
- Participants were asked to describe the differences between a solution and a diluted solution at the macroscopic, sub-microscopic, and symbolic levels.
Task 3 IAE Focus Card DA03
- Participants were asked to calculate the percentage of sugar by w/v in a Coke solution with extra ice in a tumbler.
- Participants were asked to describe what they understood about the concentration of sugar in terms of the % w/v unit at the macroscopic, sub- microscopic, and symbolic levels.
Coll and Treagust (2003a, 2003b) say using cards like this rather than just asking students questions is less threatening and helps established a more relaxed environment because the students attention is focused on the card rather than on their ability to respond to challenging questions directly.
Data Analysis
The interview data and drawings produced by the IAE protocol detailed above were analyzed thematically looking for evidence in terms of students’ understanding at the three levels of chemical representations, and their ability to relate each level to the others. Excerpts of interviews and some typical student drawings are provided below when we present the findings to show the nature of this analysis.
Research Findings and Discussion
The research findings suggest that the students’ (or users’) mental models of many aspects of dilution chemistry were generally in accord with the scientific conceptualization that is to say they did not show many alternative conceptions. However, their ability to represent the concepts at the three levels of representation varied as is detailed below.
At the macroscopic level, students were able to depict their mental models by observing the chemical phenomena in laboratory classes. At the sub-microscopic level, students were able to depict their mental models by imagination at the particulate level. At the symbolic level, students were able to depict their mental models by using of chemical symbols and mathematical formulae.
The interviewer showed the students IAE focus card: DA01. The students’ understanding was first probed by asking “Can you explain what you understand about a IM solution of sodium chloride?, and the following responses were typical of the responses obtained:
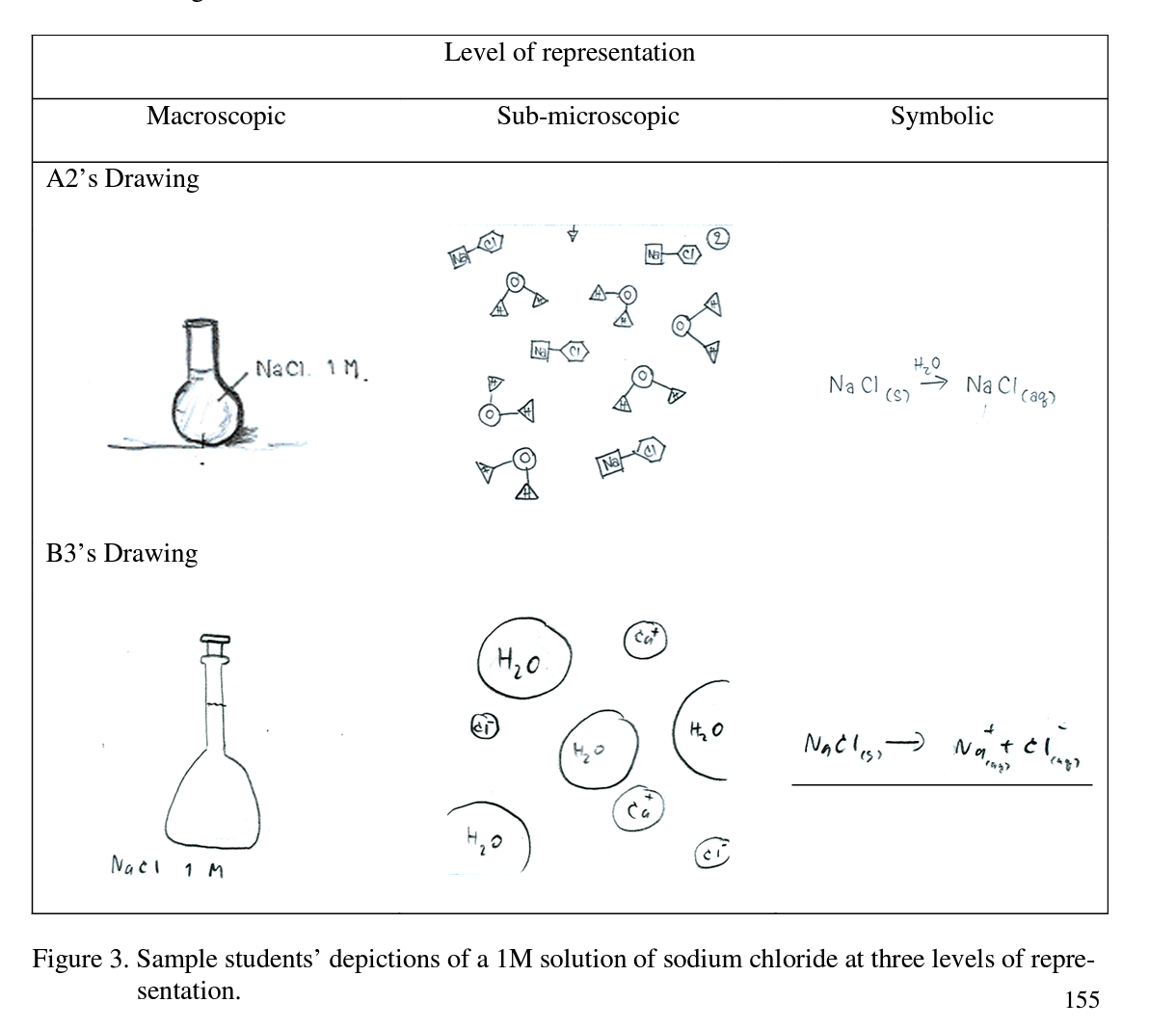
These data suggests that these students were able to successfully describe the meaning of ‘1 M of sodium chloride’. The students next were asked to depict their understanding at the three levels of representation. Solvent, solute, and homogeneity of solution were linked in the concepts as shown in Figure 3.
Two students provided detail in the interviews and talked of ionization. Student B3 pointed that NaCl is ionized in water to give Na+ and СГ ions while student A2 described NaCl in solution in the form of pairs of cations and anions, which conflicts with the scientific conceptualization (note: she also inadvertently wrote the symbol for calcium instead of sodium). This point to a molecular view of sodium chloride, which is an alternative conception.
Then interviewer next showed the students a label on a Coke bottle showing that the concentration was 10%w/v sugar, and students were asked to describe what they understood by this term. It seems that all of the students understood the meaning of a 10% w/v sugar solution.
The interviewer then showed the students IAE focus card DA02, and the students’ understanding was probed by asking “Which figure shows the most diluted Coke beverage? ”, and then asked “Why do you think that is the correct answer?”. All students provided correct answers for Figure C. Students depicted their understanding at the three levels of representation as shown in Figure 4.
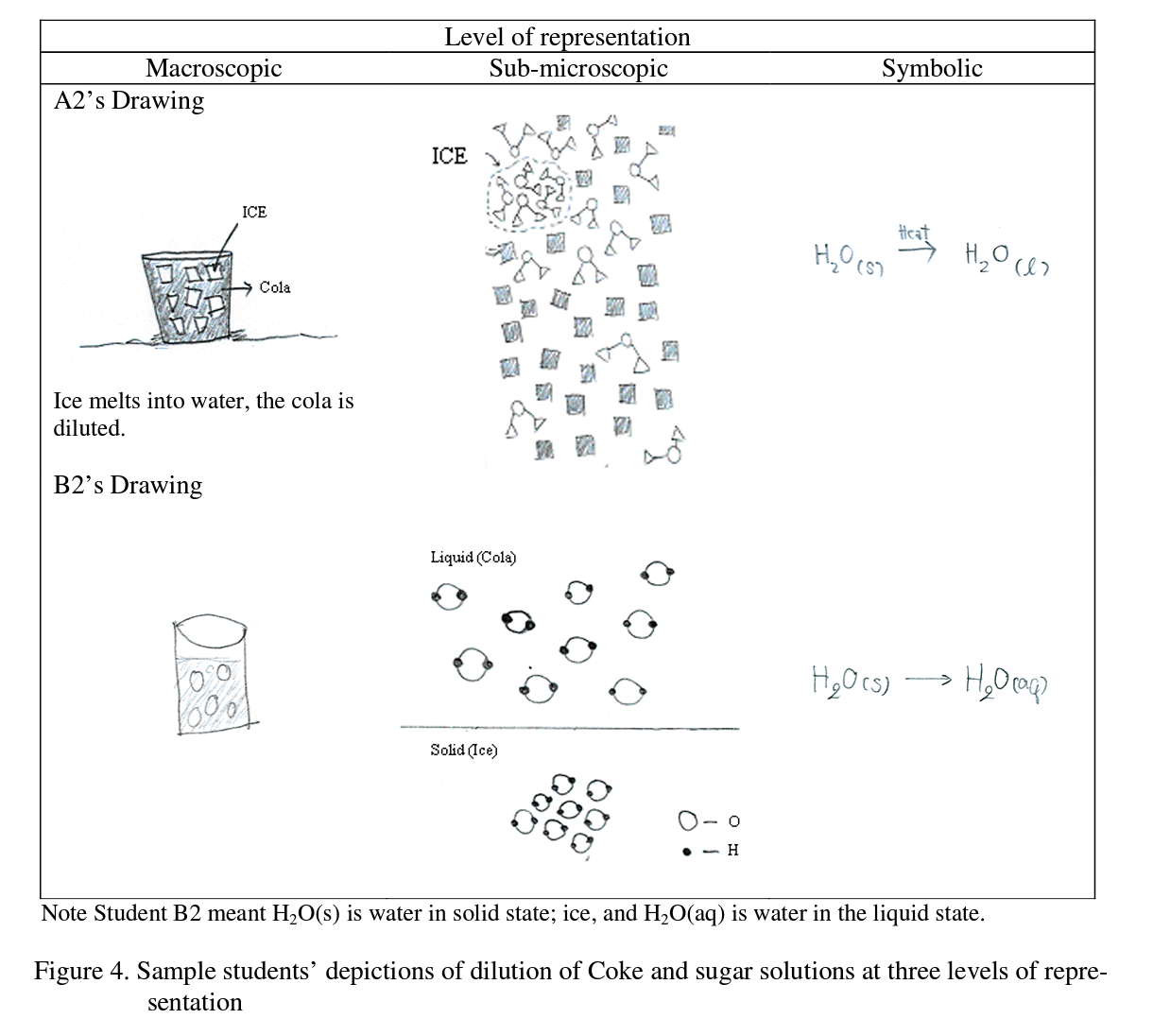
All students provided correct representations at the macroscopic level for the dilution of Coke solutions which in this case was related to their daily life (i.e., involving a familiar beverage ‘Coke’). However some of the less able students were unable to depict the diluted Coke solution at the sub-microscopic level. To further probe students’ understanding, the interviewer asked a more general question, “What is a solution?” and “What is a diluted solution?”. The following responses are typical:

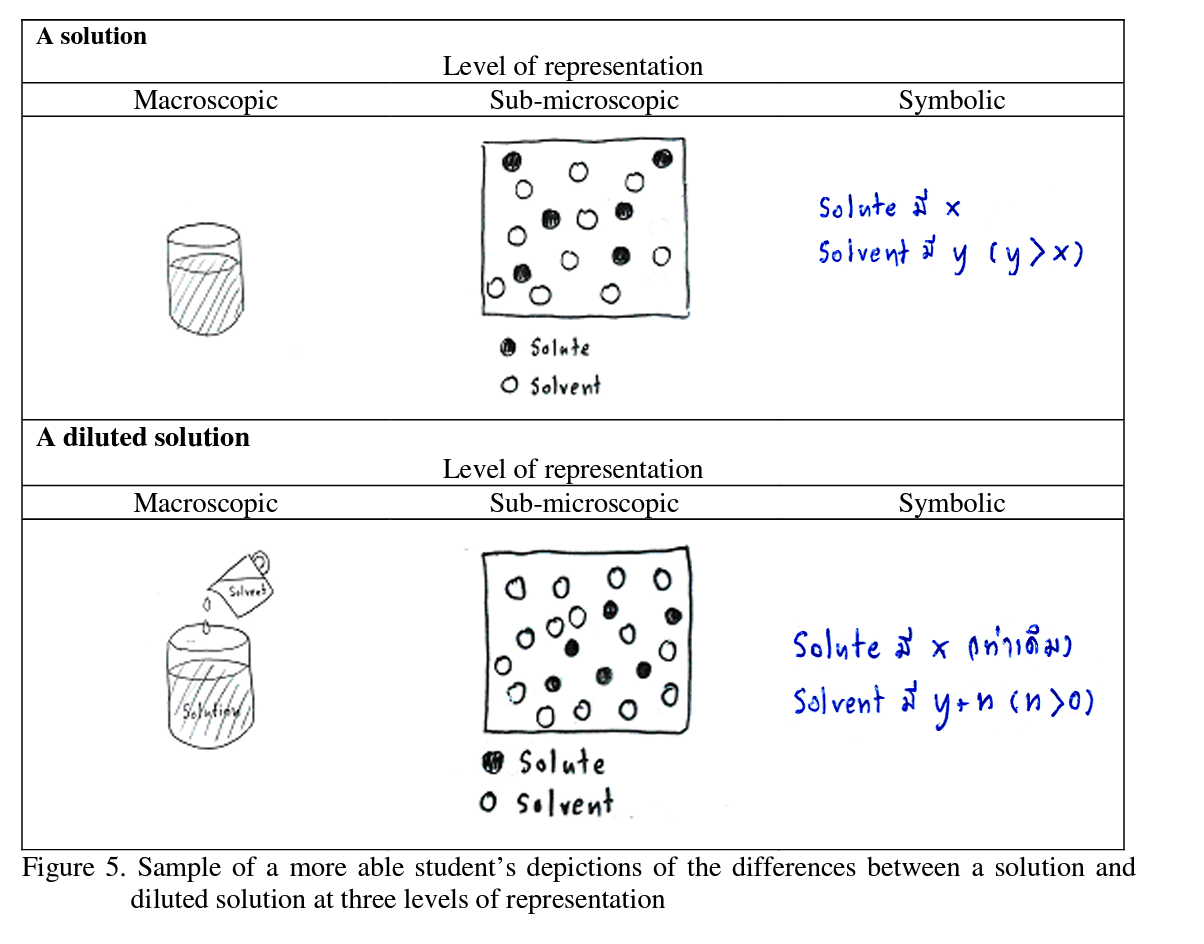
These data suggest that in this case, student A3 had a good understanding of the dilution concept. Additionally, this student was able to depict a solution and a diluted solution at each
level of representation, and identify the relationship between the levels. Hence, the more able students understood the dilution concept because they explained ‘added solvent into solution’ at the macroscopic level when they depicted by drawing of what they preformed in the laboratory classes, at the sub-microscopic level where in their drawings they depicted the numbers of solvent and solute particles, and at the symbolic level where they depicted mathematical equations:
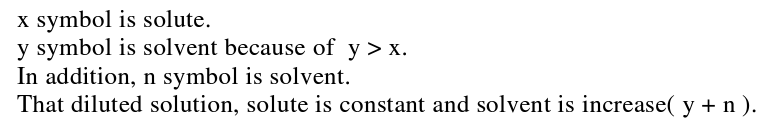
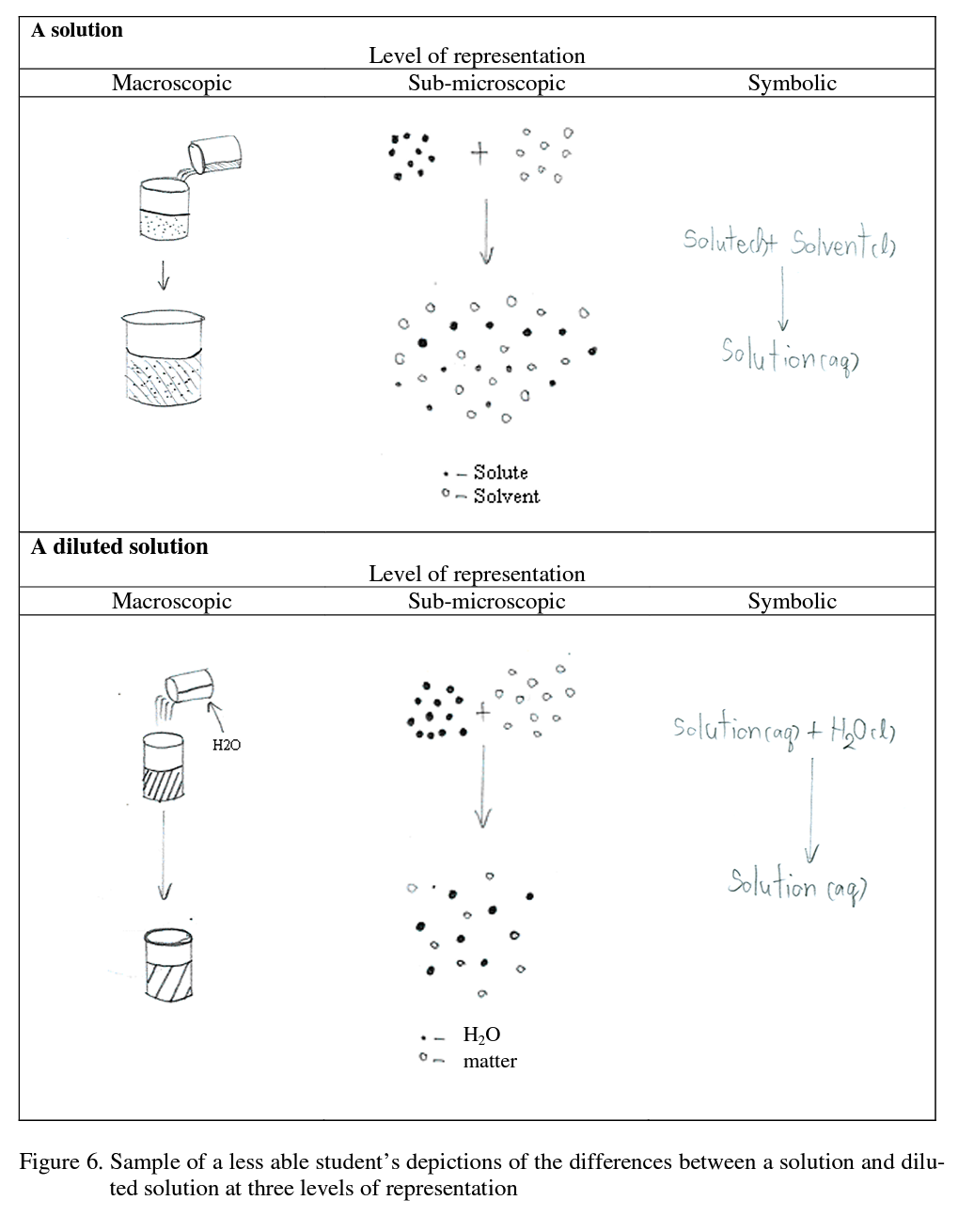
Contrast this with a less able student, student B2, whose depiction of a solution and diluted solution are presented in Figure 6:
The interviewer asked student B2 the same questions as student A3. The less able student B2 successfully depicted the nature of a solution (top part of Figure 6), the number of solvent and solute particles seems to be much the same in the diluted and undiluted solutions (see bottom part of Figure 6), meaning the student’s depiction of a diluted solution at the sub- microscopic level was not consistent with the scientific conceptualization. Likewise this student’ s depiction of dilution at the symbolic level in fact represents the solution process, rather than dilution (RHS of Figure 6). In summary, the less able students seem unsure about the dilution concept because they explained dilution as being ‘adding water into solution’ at the sub-microscopic level and by depicting the number of solvent and solute particles, but with the wrong ratio of particles. Likewise, at the symbolic level they presented dilution simply in terms of by increasing the amount of water.
Then the interviewer showed students IAE focus card DA03 and told a story about a boy who added ice to a tumbler of Coke and then drank it. Students were asked to describe what they understood based on IAE focus card DA03. The purpose of this phase of the data collection was to use an additional probe for dilution that was based on an activity very familiar to these Thai students’ everyday lives (i.e., rather than just a substance that they were familiar with).
Based on IAE focus card. The questions were classified into 4 steps.
- Pour 100 mL of Coke beverage into a tumbler.
The Coke solutions contain 10 % w/v sugar. -> 10 g/100 mL
- Added 50 mL of ice into 100 mL of Coke beverage, so we can assume the ice melted, meaning the Coke beverages were diluted, and the concentration of sugar is 10 g/150 mL.
- Assuming that students are drinking 50 mL of diluted Coke beverage in a tumbler. Then volume of solution is 100 mL. But the concentration is constant.
The concentration of sugar is 6.67 g/100 mL that it equals 10g/150 mL.
- Added 100 mL of ice into 100 mL of Coke solution, so interviewer assumed ice melted. Then Coke solutions were diluted.
Then the percentage of sugar by w/v in a Coke solution is 3.33 g/100 mL
The students were then asked to draw a depiction of this event and also asked to calculate the concentration of sugar in units of % w/v. Sample responses are shown in Figure 7
As might be expected the more able students were able to calculate the concentration of sugar in a tumbler of Coke successfully and were able to integrate between the levels of repre
sentation. At the sub-microscopic level, all students depicted things such as the amount, ratio and structure of particles in a solution. Sample depictions are shown in Figure 8.
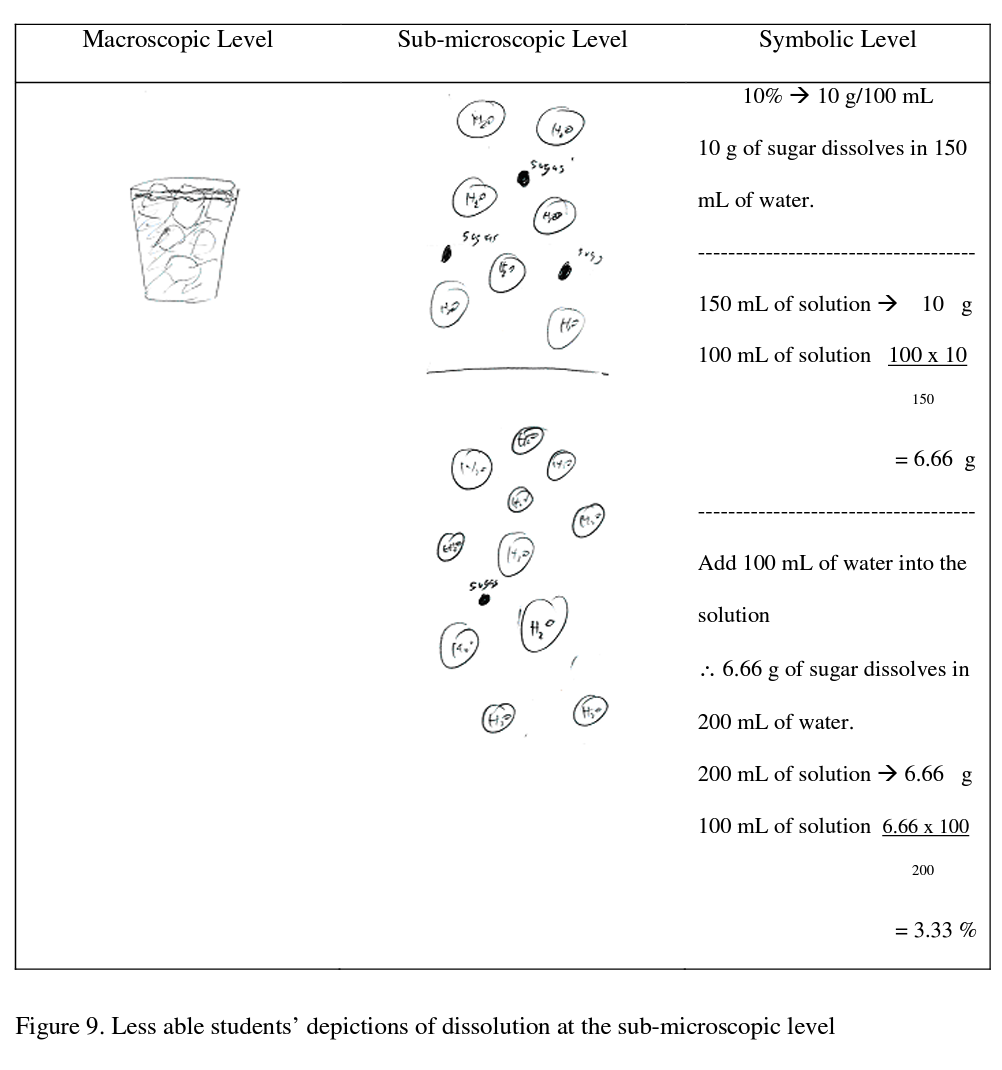
In contrast the less able students were able to depict the concentration of sugar at the symbolic level but only 1 of the 5 less able students was able to depict the concentration of sugar at the sub-microscopic level.
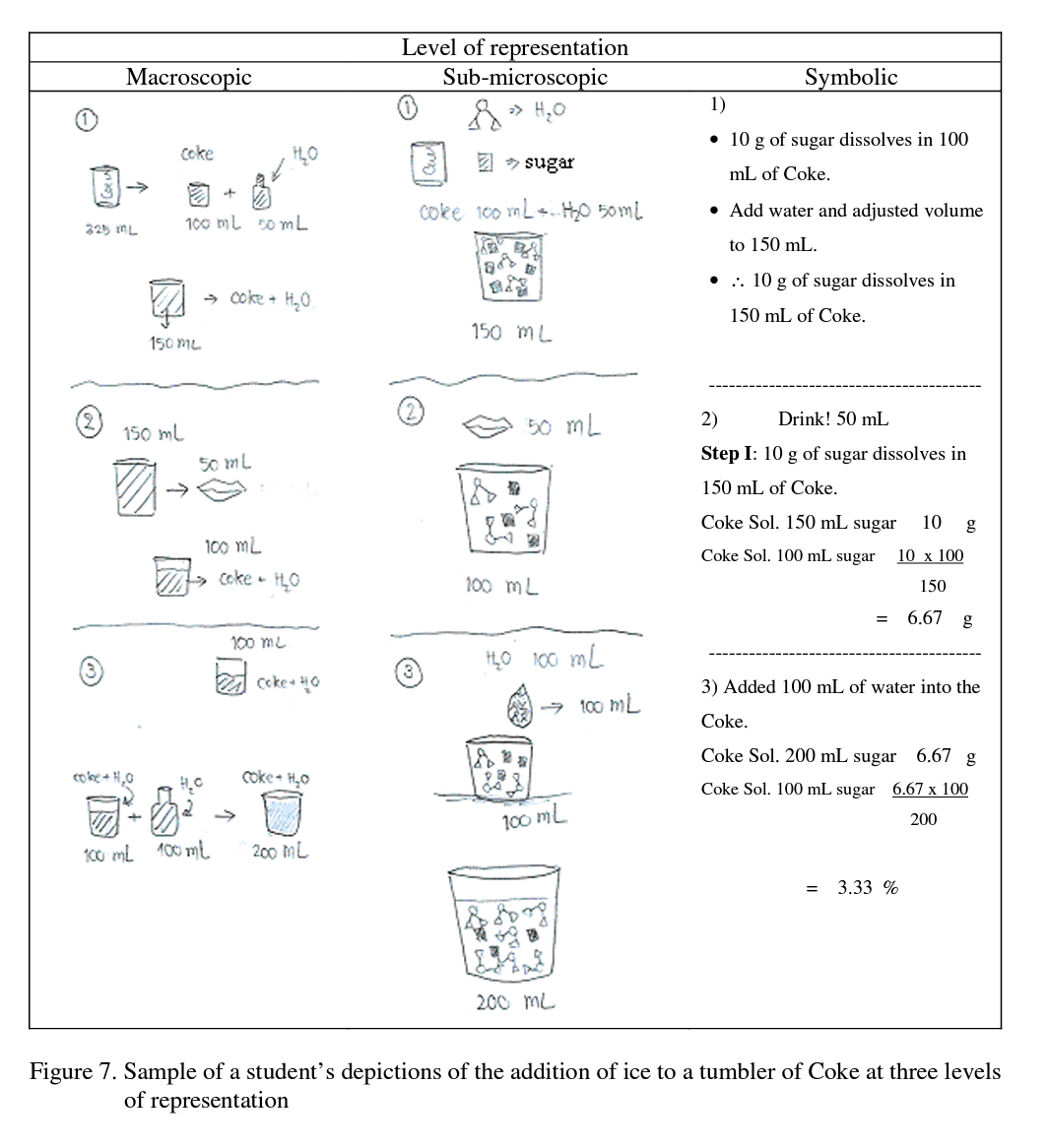

In addition, they were not able to relate the macroscopic level to the other levels. Sample drawings by less able students are shown in Figure 9.
The less able students tended to address this probe by firstly using an algorithm to do the calculation of the concentration of the solutions and then depicting the event at the sub- microscopic and macroscopic levels. Neither the sub-microscopic nor the macroscopic levels were then related to the symbolic level. As an interesting aside, dissolution of ice into water as occurs here also involves a phase change which would result in an increase in volume. This
was not noted or commented on by any of the students. We feel this is probably a consequence of the probes used, which tended to drive the students to focus on dissolution rather than changes of phase.
Discussion and Conclusions
In this work chemistry at the three levels of representations was taught to students with an intention to enhance their understanding of dilution. Their understanding of dilution was probed by several techniques: open-ended questions, drawing with description, and interviews. The findings do suggest that the interview about events approach is a useful technique to probe students’ understanding especially when the IAE focus cards with events that connected to students’ experience are used.
The more able students in the study seemed to understand the role and relationships of representations at all three levels using Johnstone’s (1991) framework. In particular, they understood the role of macroscopic and sub-microscopic levels of representation and were able to integrate into the other level as noted by (Chittieborough & Treagust, 2007) and consistent with the recommendation of Gabel (1999) and Wu et al., (2001). In contrast less able students usually presented their work mostly at the symbolic level followed by the sub-microscopic and macroscopic levels which were typically not related to the symbolic level. Hence, as might be expected students’ mental models for dilution vary, with more able students possessing more complete, relational mental models than their less able peers. This focus on the symbolic level may be related to the mode of science instruction in Thailand. Dahsah and Coll (2008) comment that in the case of stoichiometry, teachers teach using algorithms, something they argue results in superficial or shallow understanding.
Russell et al. (1997) and Treagust et al. (2003) argue that in order to understand chemistry concepts students need to be able to represent their mental models at all three of Johnstone’s (1991) levels of representation, and furthermore be able to relate these levels to each other. It seems the present work points to a need to shift students from too heavy a focus on the symbolic level and drives them to consider how the three levels relate to each other. We suggest then that the use of IAE cards and activities that use local, familiar examples are one way teachers can seek to do this. Hence, we recommend a pedagogy that involves the combination of instruction, laboratory activities development of mental models and the use of concrete models as suggested by Wu et al. (2001). Our assessment regimes then also must be consistent with such an approach and reward more than a correct numerical answer that reinforces the use of an algorithm to solve chemical problems, and fails to genuinely probe student understanding.
Acknowledgments
This work is partly supported by Institute of Innovation and Development of Learning Process (IL), Center of Excellence for Innovation in Chemistry (PERCH-CIC), the Thailand Research Fund (TRF), Mahidol University Research Grant, and Department of Chemistry, Faculty of Science, Mahidol University.
References
- Anderson, J. R. (1995). Learning and memory: An integrated approach. New york: Wiley.
- Ardac, D., & Akaygun, S. (2004). Effectiveness of multimedia-based instruction that emphasizes molecular representations on students’ understanding of chemical change. Journal of Research in Science Teaching, 47(4), 317-337.
- Beall, H., & Prescott, S. (1994). Concepts and calculations in chemistry teaching and learning. Journal of Chemical Education, 71(2), 111-112.
- Bowen, C. W. (1998). Item design considerations for computer-based testing of student learning in chemistry. Journal of Chemical Education, 75(9), 1172-1174.
- Bunce, D. M., Gabel, D. L., & Samuel, К. B. (1991). Enhancing chemistry problem-solving achievement using problem categorization. Journal of Research in Science Teaching, 28(6), 505-521.
- Qalik, M. (2005). A cross-age study of different perspectives in solution chemistry from junior to senior high school. International Journal of Science and Mathematics Education, 3(4), 671-696.
- Qalik, M., & Ayas, A. (2005). A cross-age study on the understanding of chemical solution and their components. International Education Journal, 6(1), 30-41.
- Case, J. M., & Fraser, D. M. (1999). An investigation into chemical engineering students' understanding of the mole and the use of concrete activities to promote conceptual change. International Journal of Science Education, 21(12), 1237-1249.
- Chandrasegaran, A. L., Treagust, D. F., & Mocerino, M. (2008). An evaluation of a teaching intervention to promote students’ ability to use multiple levels of representation when describing and explaining chemical reactions. Research in Science Education, 38(2), 237-248.
- Chittieborough, G., & Treagust, D. F. (2007). The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level. Chemistry Education Research and Practice, 8(3), 274-292.
- Chittieborough, G. D., Treagust, D. F., Mamiala, T. L., & Mocerino, M. (2005). Students' perceptions of the role of models in the process of science and in the process of learning. Research in Science and Technological Education, 23(2), 195-212.
- Coll, R. K. (1999). Learners’ mental models of chemical bonding. Unpublished dissertation, Curtin University of Technology, Perth, Australia.
- Coll, R. K., & Treagust, D. F. (2003a). Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding. Journal of Research in Science Teaching, 40(5), 464- 486.
- Coll, R. K., & Treagust, D. F. (2003b). Learners’ mental models of metallic bonding: A cross-age study. Science Education, 87(5), 685-707.
- Copolo, С. E., & Hounshell, P. B. (1995). Using three-dimensional models to teach molecular structures in high school chemistry. Journal of Science Education and Technology, 4(4), 295-305.
- Dagher, Z. R. (1994). Does the use of analogies contribute to conceptual change? Science Education, 78(6), 601-614.
- Dahsah, C., & Coll, R. K. (2007). Thai Grade 10 and 11 students' conceptual understanding and ability to solve stoichiometry problems. Research in Science and Technological Education, 25(2), 227- 241.
- Dahsah, C., & Coll, R. K. (2008). Thai Grade 10 and 11 students' understanding of stoichiometry and related concepts. International Journal of Science and Mathematics Education, 6(3), 573-600.
- Devetak, I. (2005). Explaining the latent structure of understanding submicropresentations in science. Unpublished dissertation, University of Ljubljana, Slovenia.
- Devetak, I., Urbancic, M., Wissiak Grm, K. S., Kmel, D„ & Glazar, S. A. (2004). Submicroscopic representations as a tool for evaluating students’ chemical conceptions. Acta Chimica Slovenica, 51,799-814.
- Devetak, I., Vogrinc, J., & Glazar, S. A. (in press). Assessing 16-Year-Old students’ understanding of aqueous solution at submicroscopic level. Research in Science Education.
- Dori, Y. J., & Hameiri, M. (2003). Multidimensional analysis system for quantitative chemistry problems: Symbol, macro, micro, and process aspects. Journal of Research in Science Teaching, 40(3), 278-302.
- Duit, R. (1991). On the role of analogies and metaphors in learning science. Science Education, 75(6), 649-672.
- Dunnivant, F. M., Simon, D. M., & Willson, S. (2002). The making of a solution: A simple but poorly understood concept in general chemistry. The Chemical Educator, 7(4), 207-210.
- Famham-Diggory, S. (1994). Paradigms of knowledge and instruction. Review of Educational Research. 64(3), 463-477.
- Foshay, R., & Kirkley, J. (2003). Principles for Teaching Problem Solving. Technical Paper (No. PLATO-TP-4). U.S. Minnesota
- Franco, C., & Colinvaux, D. (2000). Grasping mental models. In J. K. Gilbert & C. J. Boulter (Eds.), Developing models in science education (pp. 93-118). Dordrecht: Kluwer
- Gabel, D. L. (1993). Use of the particle nature of matter in developing conceptual understanding. Journal of Chemical Education, 70(3), 193-194.
- Gabel, D. L. (1999). Improving teaching and learning through chemistry education research: A look to the future. Journal of Chemical Education, 76(4), 548-554.
- Gilbert, J. K., Boulter, C. J., & Rutherford, M. (2000). Explanations with models in science education. In J. K. Gilbert & C. J. Boulter (Eds.), Developing models in science education (pp. 193-208). Dordrecht: Kluwer.
- Gilbert, J. K., Watts, D. M., & Osborne, R. J. (2005). Eliciting student views using an interview-about- instances technique. In J. K. Gilbert (Ed.), Constructing worlds through science education: The selected works of John K. Gilbert. London: Routledge.
- Gobert, J. D., & Buckley, В. C. (2000). Introduction to model-based teaching and learning in science education. International Journal of Science Education, 22(9), 891-894.
- Greca, I. M., & Moreira, M. A. (2000). Mental models, conceptual models, and modelling. International Journal of Science Education, 22(1), 1-11.
- Grosslight, L., Unger, C., Jay, E., & Smith, C. (1991). Understanding models and their use in science: Conceptions of middle and high school students and experts. Journal of Research in Science Teaching, 28(9), 799-822.
- Harrison, A. G., & Treagust, D. F. (2000). Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in grade 11 chemistry. Science Education, 84(3), 352-381.
- Heyworth, R. M. (1999). Procedural and conceptual knowledge of expert and novice students for the solving of a basic problem in chemistry. International Journal of Science Education, 21(2), 195- 121.
- Hinton, M. E., & Nakhleh, M. B. (1999). Students' microscopic, macroscopic, and symbolic representations of chemical reactions. The Chemical Educator, 4(4), 1-29.
- Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Learning, 7,75-83.
- Johnstone, A. H. (1993). The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9), 701-705.
- Jonassen, D., & Tessmer, M. (1996). An outcome-based taxonomy for instructional systems design, evaluation and research. Training Research Journal, 2,11-46.
- Kozma, R. B., & Russell, J. (1997). Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. Journal of Research in Science Teaching, 34(9), 949-968.
- Lythcott, J. (1990). Problem solving and requisite knowledge of chemistry. Journal of Chemical Education, 67(3), 248-252.
- McElroy, L. J. (1996). Teaching dilutions. Journal of Chemical Education, 73(8), 765-766.
- Mulford, D. R., & Robinson, W. R. (2002). An inventory for alternate conceptions among first- semester general chemistry students. Journal of Chemical Education, 79(6), 739-744.
- Norman, D. A. (1983). Some observations on mental models. In D. A. Gentner & A. L. Stevens (Eds.), Mental models (pp. 7-14). Hillsdale, NJ: Lawrence Erlbaum Associates.
- Nurrenbem, S. C., & Pickering, M. (1987). Concept learning versus problem solving: Is there a difference? Journal of Chemical Education, 64(6), 508-510.
- Pinarbasi, T., & Canpolat, N. (2003). Students’ understanding of solution chemistry concepts. Journal of Chemical Education, 80(11), 1328-1332.
- Pittman, К. M. (1999). Student-generated analogies: Another way of knowing? Journal of Research in Science Teaching, 36(1), 1-22.
- Raviolo, A. (2001). Assessing students’ conceptual understanding of solubility equilibrium. Journal of Chemical Education, 78(5), 629-631.
- Robinson, W. R. (2003). Chemistry problem-solving: Symbol, macro, micro, and process aspects. Journal of Chemical Education, 80(9), 978-982.
- Russell, J. W., Kozma, R. B., Jones, T., Wykoff, J., Marx, N., & Davis, J. (1997). Use of simultaneous- synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts. Journal of Chemical Education, 74(3), 330-334.
- Savelsbergh, E. R., De Jong, T., & Ferguson-Hessler, M. G. M. (2002). Situational knowledge in physics: The case of electrodynamics. Journal of Research in Science Teaching, 39(10), 928-951.
- Schmidt, H. J., & Jigneus, C. (2003). Students' strategies in solving algorithmic stoichiometry problems. Chemistry Education: Research and Practice, 4(3), 305-317.
- Staver, J. R., & Lumpe, A. T. (1995). Two investigations of students' understanding of the mole concept and its use in problem solving. Journal of Research in Science Teaching, 32(2), 177-193.
- Tasker, R., & Dalton, R. (2006). Research into practice: Visualisation of the molecular world using animations. Chemistry Education Research and Practice, 7(2), 141-159.
- Treagust, D. F. (1993). The evolution of an approach for using analogies in teaching and learning science. Research in Science Education, 23,293-301.
- Treagust, D. F., Chittieborough, G., & Mamiala, T. L. (2003). The role of submicroscopic and symbolic representations in chemical explanations. International Journal of Science Education 25(11), 1353-1368.
- Ünal, S., Qalik, M., Ayas, A., & Coll, R. K. (2006). A review of chemical bonding studies: Needs, aims, methods of exploring students’ conceptions, general knowledge claims and students’ alternative conceptions. Research in Science and Technological Education, 24(2), 141-172.
- Van Der Veer, C. G., & Del Carmen Puerta Melguizo, M. (2003). Mental models. In J. A. Jacko & A. Sears (Eds.), The human-computer interaction handbook: Fundamentals, evolving technologies, and emerging applications (pp. 52-80). Uitgever: Lawrence Erlbaum & Associates.
- Venville, G., Bryer, L., & Treagust, D. F. (1994). Training students in the use of analogies to enhance understanding in science. Australian Science Teachers Journal, 40(60-68).
- Wang, M. R. (2000). An introductory laboratory exercise on solution preparation: A rewarding experience. Journal of Chemical Education, 77(2), 249-250.
- Wu, H.-K., Krajcik, J. S., & Soloway, E. (2001). Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom Journal of Research in Science Teaching, 38(7), 821-842.





