Promoting Scientific Literacy Using a Sociocritical and Problem-Oriented Approach to Chemistry Teaching: Concept, Examples, Experiences


Опубликована Июль 10, 2009
Последнее обновление статьи Янв. 1, 2023
Abstract
This paper revisits the discussion about the objectives of scientific literacy-oriented chemistry teaching, its connection to the German concept of Allgemeinbildung, and the debate of science through education vs. education through science. About 10 years ago the sociocritical and problem-oriented approach to chemistry teaching was suggested using these starting points. In this paper its central assumptions and criteria for structuring lesson plans are presented as they have been refined along a series of lesson plans developed by participatory action research in recent years. The summarized teaching approach intends to more thoroughly promote reflection on scientific questions in the framework of their socioeconomical and ecological consequences. This is done by inserting authentic and controversial debates on socioscientific issues into chemistry teaching, which provoke and allow for open discussions and individual decision making processes. After discussing the framework, we present one example which deals with musk fragrances used in cosmetic products, and we give an overview of different respective issues. From experience gained in applying the different examples, the potential of this teaching approach is then reflected upon as a source for promoting the process-oriented skills of evaluation and communication as essential parts of a welldeveloped scientific literacy.
Ключевые слова
STS, scientific literacy, sociocritical and problem-oriented chemistry teaching
Introduction and Legitimation of a New Approach to Chemistry Teaching, Or: Scientific Literacy, Allgemeinbildung, and ‘Education through Science’
Chemistry classes at the secondary level are unpopular among students in Germany (Gräber, 2002), just as they are in other European countries and the USA (Black & Atkin, 1996; Osborne 2003). Added to its unpopularity, German chemistry teaching has quite often been characterized as ineffective in promoting higher-order cognitive skills, such as students’ skills in communication or in evaluating socioscientific issues (Gräber, 2002; Fischer et al., 2005). One reason for this unpopularity, and the low success rates in achieving high-order cognitive skills, is believed to be the fact that most chemistry lessons use an overly content-driven approach.
This approach appears to be too oriented towards the inner systematics of chemistry (Gräber, 2002). In the students’ opinion, such chemistry classes lack personal relevance for them, which leads to both low levels of motivation and also a general lack of interest in chemistry (Morell & Lederman, 1998; Osborne, 2007; Osborne, Driver & Simon, 1998). German chemistry teaching is not sufficiently oriented towards problem-solving and practical applications (Stanat et al., 2002). Therefore, chemistry teaching does not focus enough on the interplay of science, technology and society with regard to local issues, public policy-making and global problems (Gräber, 2002; Eilks, 2000; Eilks, Marks & Feierabend, 2008). This remains the case, despite science educators repeatedly indicating the need to make students competent in socioscientific reasoning and to prepare young people to participate in socioscientific controversies. Such changes must occur if teaching is to focus on the development of scientific literacy in its learners (e.g., Bybee, 1997; Driver, Leach, Millar & Scott, 1996; Eilks, 2000; Holbrook, 2003; Osborne, 2007; Pedretti & Hodson, 1995). Holbrook and Rannikmäe (2007, p. 1347) comment thus:
Science education should be regarded as “education through science”, rather than “science through education”. (...) This encompasses an understanding of the nature of science [education], with links to achievement of goals in the personal domain, stressing intellectual and communication skill development, as well as the promotion of character and positive attitudes, plus achievement of goals in the social education domain, stressing cooperative learning and socio-scientific decision-making. [...] the over-riding target for science teaching in school, as an aspect of relevant education, is seen in responsible citizenry, based on enhancing scientific and technological literacy.
We totally agree with this position. In our opinion, one promising way to help students close the gap between school science, applications of science and technology and their critical evaluation can be brought about by designing chemistry lessons to include societal issues and discussions involving science and technology (Albe, 2008; Holbrook, 1998; Ratcliffe, 1998). However, the selection of such everyday-life contexts of chemistry and technology should not be arbitrary. Issues should be chosen which are authentic and truly relevant for students’ lives. Numerous arguments support this idea. Many of these stem from viewing science education more thoroughly from the perspective of activity theory (Roth & Lee, 2004; Van Aalsvoort, 2004a, 2004b). Activity theory demands that science education be oriented towards students’ personal needs and interests in order to increase the relevance of science education in the eyes of the students (Fensham, 2004; Holbrook & Rannikmäe, 2007). This must, however, be accomplished without neglecting the attainment of a basic understanding of relevant science concepts. Such understanding is necessary both for identifying key scientific issues and also for engaging students in appropriate socioscientific discussions based on well-grounded knowledge (Lewis & Leach, 2006).
The same legitimation also can be obtained from the German teaching tradition, which defines the main objective of schooling as achieving a high level of Allgemeinbildung (general education). This word encompasses several main goals of education, namely the development of competency in: 1) self-determination (Selbstbestimmungsfähigkeit), meaning that the individual learns how to both accept responsibility for and successfully represent his/her own interests within society, 2) active engagement in the positive development of a democratic society through consensus (Mitbestimungsfähigkeit), and 3) showing solidarity with others (Solidaritätsfähigkeit) (see e.g., Klafki, 2000). Roth and Lee (2004) and Elmose and Roth (2005) presented the German Allgemeinbildung-tradition in an international forum on the focus of education. They characterised Allgemeinbildung as a readiness for both life and participation
in a modern society. With regard to education, we must unequivocally state that such societies are strongly based on science and technology. It is clear that such an approach focuses - aside from learning scientific concepts, facts and applications - very strongly on the general goals of education. In a nutshell, the idea is not only to promote the learning of science in the sense of ‘science through education’, but also to promote ‘education through science’ as reported above in the quote from Holbrook and Rannikmäe (2007). Such an understanding of education through science demands structures which promote communication and evaluation skills that can be applied within science, but also beyond, from chemistry education. These skills are necessary to reflect the interplay of science and technology with society, ecology, economy, and with learners' own desires, needs and interests (e.g., Aikenhead, 2007; Bybee, 1987; Fensham, 2004; Gräber, 2002; Solomon & Aikenhead, 1994).
Such reforms in science teaching have been repeatedly demanded by various groups and individuals. Science education should both promote a broader view of science, while simultaneously helping to foster an appreciation for science and its usefulness to society (e.g., Bybee 1987; Solomon & Aikenhead, 1994; Osborne, Driver & Simon, 1998; Osborne, 2001; Millar, 2006; Roberts, 2007; Holbrook & Rannikmäe, 2007). Moreover, Bybee (1997, p. 61) described such interactions as the core issue for well-developed, multidimensional scientific literacy: “The learner makes connections within the science disciplines, between science and technology, and between science and technology and larger social problems and aspirations.”
Before, during and after Bybee’s well-recognized contributions in the 1980s and 1990s (see e.g., Bybee, 1987, 1997) there were extensive discussions of such aspects as: How to make science teaching more relevant to students, how to promote competency in evaluating socio-scientific issues as a central objective of science lessons, and how to teach students about the inter-relatedness of science, technology and society. Actual overviews of the STS- movement are given in Sadler’s review (2004) or in the framework of the critical discussion of its origins and the development of the terms scientific literacy and science literacy by Roberts (2007).
Such STS-oriented chemistry lessons include a reflective overview of chemistry, its industrial applications and its ecological and socioeconomic impacts. STS education is considered as necessary, if education is understood as a process of creating literate citizens who are able to play an active and responsible role in democratic decision-making processes, and also participate in discussions about developments based on science and technology and their potential impacts (e.g., Holbrook & Rannikmäe, 2007; Millar, 1996). In addition, this approach may also improve students’ interest in and attitudes towards science lessons (e.g., Lee & Erdogan, 2007; Millar, 2006; Osborne, et al., 1998), aspects which are of great importance for learning achievement (Simpson, Koballa, Oliver & Crawley, 1994).
Outline of a New Teaching Concept, Or: The Sociocritical and Problem-Oriented Approach to Chemistry Teaching
Taking the above-mentioned framework into account, Eilks described a new conceptual approach to chemistry teaching in Germany about 10 years ago (see e.g., Eilks, 2000, 2002a). His example was based on the ecological evaluation of biodiesel usage, and the approach was titled A Sociocritical and Problem-Oriented Approach to Chemistry Teaching. More detailed descriptions of the approach were discussed step-by-step and refined in a series of earlier papers in the German or English language (e.g., Eilks, 2002a, Marks, Bertram & Eilks, 2008, Eilks, Marks & Feierabend, 2008). Our current conceptual framework was developed side by
side with this and contains many parallels to the Scientific and Technological Literacy for All (STL) approach as outlined by Holbrook from the late 1990s (e.g., Holbrook, 1998, 2003).
The sociocritical and problem-oriented approach to chemistry teaching aims at promoting students’ motivation, bettering their attitudes towards chemistry and chemistry teaching and achieving a broad range of educational goals (e.g., Eilks, Marks & Feierabend, 2008). The main foci of different approaches to chemistry teaching were defined in the beginning as follows:
- to increase students’ interest in science and technology and to reveal the relevance of science in societal discussions and decision-making;
- to make students aware of their own personal interest and motivate them to promote and protect their self-interest (either as consumers or within political decision-making processes); to provoke and develop decision-making processes within the individual;
- to promote students’ skills in the critical use of information and increase their selfreflection on why, when and how science-related information is used by effected groups and/or for public purposes; and
- to promote student-active science learning which is motivated using relevant, current and controversial socio-scientific issues.
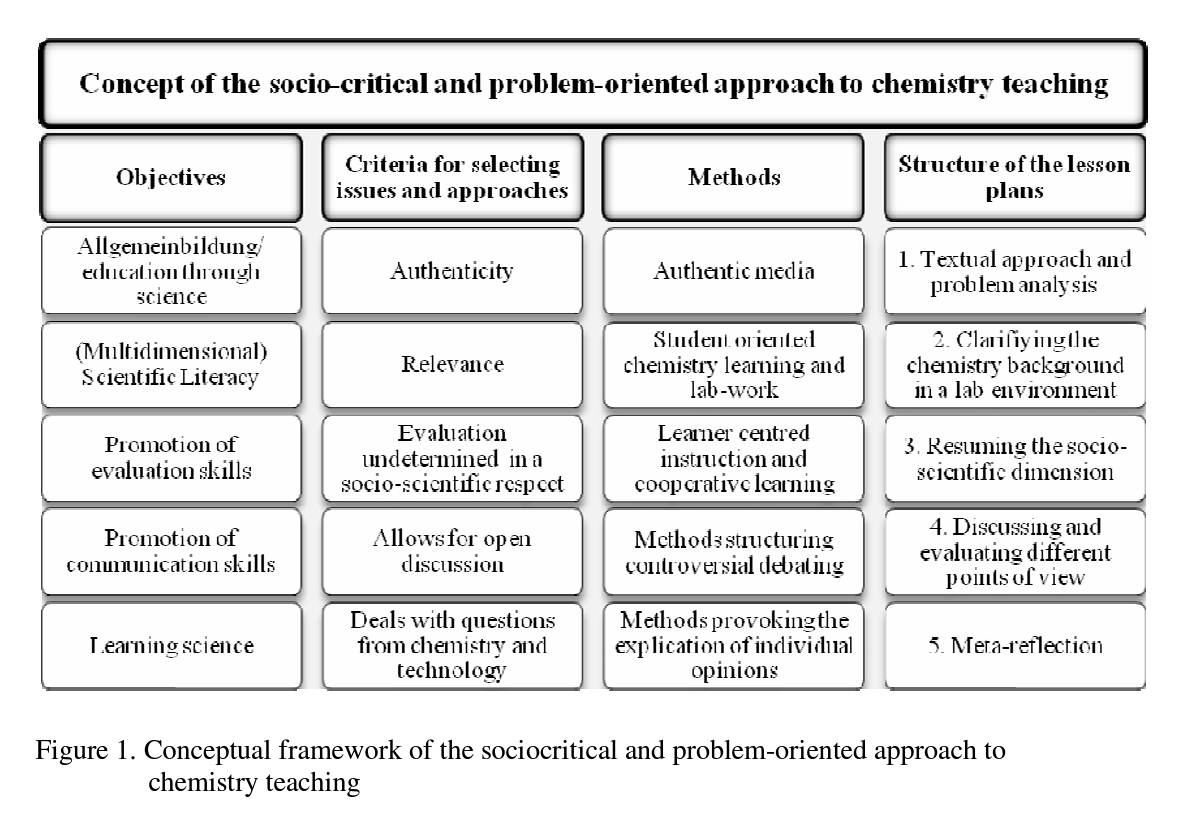
By developing various examples we subsumed several key elements of our socio-critical and problem-oriented approach into chemistry lesson planning and created a common structure for the lesson plans (e.g., Eilks, Marks & Feierabend, 2008): Potentially useful socio- scientific issues must meet specific criteria to fulfill our teaching intentions. Our lesson plans always start with authentic, current and controversial problems being debated within society. These topics must be present in different media sources, such as newspaper articles, brochures from pressure groups, advertisements, reports on TV, and so on, which are used to introduce the lesson plan and provoke a first round of questions and discussions. Only issues allowing authentic differences of opinion which have been expressed in public debate by different stakeholders or pressure groups are chosen. Inappropriate issues are those which allow only one-sided solutions or those which would be viewed as unacceptable due to scientific, ethical, or sociological reasons by a majority of the class, teachers or parents. Additionally, only issues which allow open decision-making processes are selected. The teaching activities challenge students to make up their own minds and express their opinions in an open forum. This method ensures that learners can express their personal points-of-view without judgment, censorship or condemnation as outsiders by the rest of the group or the teacher. Nevertheless, all lesson plans include and teach basic chemistry theory. They are built on a foundation of student lab-work and the use of open methods of learner-centered instruction, such as cooperative learning forms like the ‘jigsaw’ classroom (e.g., Marks, Bertram & Eilks, 2008) or the ‘learning at stations’ method (e.g., Eilks, 2002b). Discussion techniques are used to draw out different points of view, to recognize how contrary these can be, and to see how such opinions are presented, promoted and manipulated within society at large. Figure 1 gives a conceptual overview of the teaching approach.
In short, the teaching approach must start with societally-relevant, current, authentic and controversial issues from within society. These topics must have the potential to allow the learning of basic chemistry content knowledge, while simultaneously opening up group discussions and promoting open decision-making processes. This is in line with Sadler (2004, p.
523), who described the most fruitful settings for science education as: “those which encourage personal connections between students and the issues discussed, explicitly address the value of justifying claims and expose the importance of attending to contradictory opinions.”
An Example from the Classroom, Or: Reflecting About Musk Fragrances in Shower Gels
The following example illustrates how relevant issues can be found and how a socio-critical and problem-oriented chemistry lesson plan is structured. A highly-controversial topic which is currently being debated at various levels of society is the use of specific fragrances, namely synthetic musks, in cosmetic products (Marks, Witte & Eilks, 2007; Marks & Eilks, 2008c). Bester (2007) gives a concise overview of synthetic musk fragrances and their presence in and effects on the environment. The main problem causing the present dilemma is that synthetic musk fragrances are produced as high volume chemicals in volumes of over 2,000 tons a year in Western Europe (EU). They are used as perfumes in a wide range of cosmetic products. Synthetic musk fragrances are cheap to produce and are indispensable for the body care and detergent/soap industries, since they easily attach themselves to the surface of the skin. We divide synthetic musk fragrances into three different groups: nitro, polycyclic and macrocyclic musk fragrances. In recent years the suspected cancer-causing nitromusks have been almost completely replaced by polycyclic compounds. The commercially most important polycyclic musks are HHCB and AHTN (trade names Galaxolide and Tonalide) which, when taken together, control a market percentage of over 95%. But these compounds are not unproblematic for the environment. Synthetic musk fragrances are funneled into wastewater systems in great volumes via private homes and industrial concerns, thanks to widespread use of cleaners and body care products (Artola-Garicano, Borknent, Kennens & Vaes, 2003). А large amount of these substances pass through sewage treatment plants largely chemically unaltered, before they are discharged (largely intact) into streams, rivers and lakes (Simonich, et al., 2002). Therefore, the concentration of synthetic musk fragrances is noticeably high near discharge points of municipal water clarification plants (Eilks & Bester, 2003). Furthermore, synthetic polycyclic musks are easily stored in the fatty tissues of aquatic organisms due to their lipophilic nature, especially in the tissues of oily fishes (Eschke, Dibowski & Traud, 1995a; Ga- terman, et al., 2002; Hajslova Gregor, Chadlova & Alterova, 1998). This is problematic because both of the most important fragrances, Galaxolide and Tonalide, have shown hormone- activating effects and may lead to falling levels of fertility in male fish (Bester, 2007; Seinen, Lemmen, Pieters, Verbruggen & van der Burg, 1999). This effect has not yet been documented in humans. But synthetic musks have already been detected stored in human tissue samples and, maybe even more problematically, in human breast milk (Duedahl-Olesen, Cederberg, Pedersen & Hojgard, 2005; Eschke, Dibowski & Traud, 1995b). Researchers fear that these fragrances might have similar effects in the human body (Müller, Schmidt & Shlatter, 1996). Aside from an accumulation problem via nature and the food chain, a second problem stems from the processing of the sludge produced by wastewater facilities, since this sludge is not infrequently contaminated with synthetic musk fragrances. It is used in agriculture and is therefore introduced directly into our personal environments (Simonich et al., 2002). At present macrocyclic musk fragrances, which are allegedly more environmentally friendly, are being introduced into the market. However, there have not yet been sufficient analytical tests performed upon these substances to prove or disprove these claims (e.g., Bester, 2007). Until now there have been almost no legislative regulations concerning the use of synthetic musk fragrances. There is no way for consumers to discern whether the products they purchase contain synthetic musk fragrances (and which type) or not, because lists of detailed product ingredients are optional and normally not a legal requirement. However, consumer tests of different products can clarify this question for perspective buyers. For example the German journal Ökotest, a magazine testing consumer products with respect to their health and environmental effects, used the presence of synthetic musk fragrances as a ‘weed-out factor’ in its testing of shower gels. Products containing such substances cannot receive scores rating them ‘good’ or better (Ökotest, 2004).
The synthetic fragrance scenario was used to develop a lesson plan for German 10th grade (age-range 15-16) chemistry lessons (Marks, Witte & Eilks, 2007) within a project of participatory action research (Eilks & Ralle, 2002; Eilks, Marks & Feierabend, 2008). The lesson plan consisted of 8-10 forty-five minute classroom periods. The embedded basic chemistry knowledge about detergents and their function is part of the official governmental syllabus in Germany for this grade level.
In the initial lesson of this lesson plan project, various shower gels are presented to the pupils. The products should retain their price tags and include supermarket items, products from discounters or ‘dollar stores’, brand-name and generic articles, and examples of shower products without fragrances and/or preservatives. The students are challenged to select one of the products to use and list their reasoning on slips of paper. The reasons are then clustered on the blackboard into groupings containing similar arguments. In each testing cycle of this introductory lesson (either with student teachers at the university or with students in school), a vast majority of the groups mentioned the smell of the product as the leading criterion for their choice. This selection criterion is always followed by the image of the product and the appearance of the packaging. Reasons mentioning good functionality as a detergent, skin care ingredients or other reasons (e.g., pH-neutrality/hypoallergenic) are only rarely mentioned. The criteria of the students are compared to an authentic text taken from a consumer test magazine (Ökotest, 2004), leading to a discussion about the major components of shower gels (detergents, skin care ingredients, fragrances, dyestuffs and preserving agents). Then comes questions about which of these substances are actually necessary for a shower gel to carry out the tasks to clean and care for the skin, which ones are added for other reasons, and which may be accounted for when evaluating a product. The text makes clear that especially the latter groups of substances (fragrances, dyestuffs and preserving agents) differentiate between the various products and are of primary importance for evaluating products concerning their health or environmental effects (see above).
The explanation of the basic chemistry behind the function of a shower gel and its ingredients takes place through a learning-at-stations setting (e.g., Eilks, 2003). Within this leam- ing-at-stations approach, the learners work for 2-3 classroom periods (each of 45 min. duration) to finish a total of eight learning stations and their content. The stations are offered in the classroom and contain different activities, such as experiments, texts or modeling tasks. Students are allowed to divide the time at their disposal among the different stations using their own judgment and to decide the sequence of visiting the different stations while working in small groups of 3 to 4 students. Of a total of eight stations, three deal with detergents as a main ingredient of functional compounds in a shower gel. Three others deal with fragrances. Two further stations clarify ‘other’ ingredients and include the making of a shower gel by the students themselves. Five of the eight stations include easy hands-on experiments, one uses graphic animation, one is text-based and one is model-based. For especially-talented or rapidly working groups, two extra stations with in-depth information on detergent types (detergent types (structures and classification of anionic, cationic, zwitterionic, or nonionic surfactants) ,and the synthesis of fragrances are offered (Marks & Eilks, 2008c; Marks, Witte & Eilks, 2007).
In order to link newly-learned chemistry knowledge with the problematics of using synthetic musks, an overview of how fragrances are extracted and then used by perfume makers is provided in a film or text. Also, the initial text from the consumer test magazine is revisited. In order to introduce controversy through different and/or partially contradictory views on the topic, the students are asked to produce a news report on the issue as if they were journalists working for a television program. The students receive various news tickers as a source of information, much like journalists would take their information from news agencies' summaries. Within each of the various news tickers, a set of short messages is offered which discuss the issue from different perspectives using different kinds of resources (e.g., from companies, pressure groups, and scientists). The different news tickers cover the viewpoints of 1) consumer protection agencies (i.e., concerns about human contact with potentially hormone- activating and allergenic substances), 2) the cosmetics industry (i.e., production and marketing of a competitive product), 3) environmental protection groups (i.e., effects of synthetic musk fragrances in/on nature), and 4) the waste water treatment community (i.e., problems and costs for proper wastewater treatment and clarification) (Marks & Eilks, 2008b).1 Two independent groups of 2-3 pupils are assigned to each perspective to ensure that all four perspectives are covered by two teams. The purpose here is to sensitize the students to the fact that information taken from the exact same sources can be presented differently by two different ‘journalists’, and also to show how varied the resulting news can be. The students should also be made aware of the role that a journalist’s subject knowledge plays when writing a report on a scientific evaluation of a product. Pupils must also be creative. They must carefully evaluate and choose journalistic reporting ‘tricks’ which are necessary to attract large audiences to the news, how much information can effectively be presented in one minute and how much less- important or background information is needed - and often is used by journalists - for reporting on a complex topic. Finally, the freshly-created news clips are presented to and evaluated by the entire class as to their comprehensibility, presentation and content information. A metadiscussion at the end of the lesson reveals the differences in the perspectives, including their relevance and connections to the interests of different shareholder groups in society, and reflects upon how complex such a simple question like, Which shower gel should I buy or use? can become.
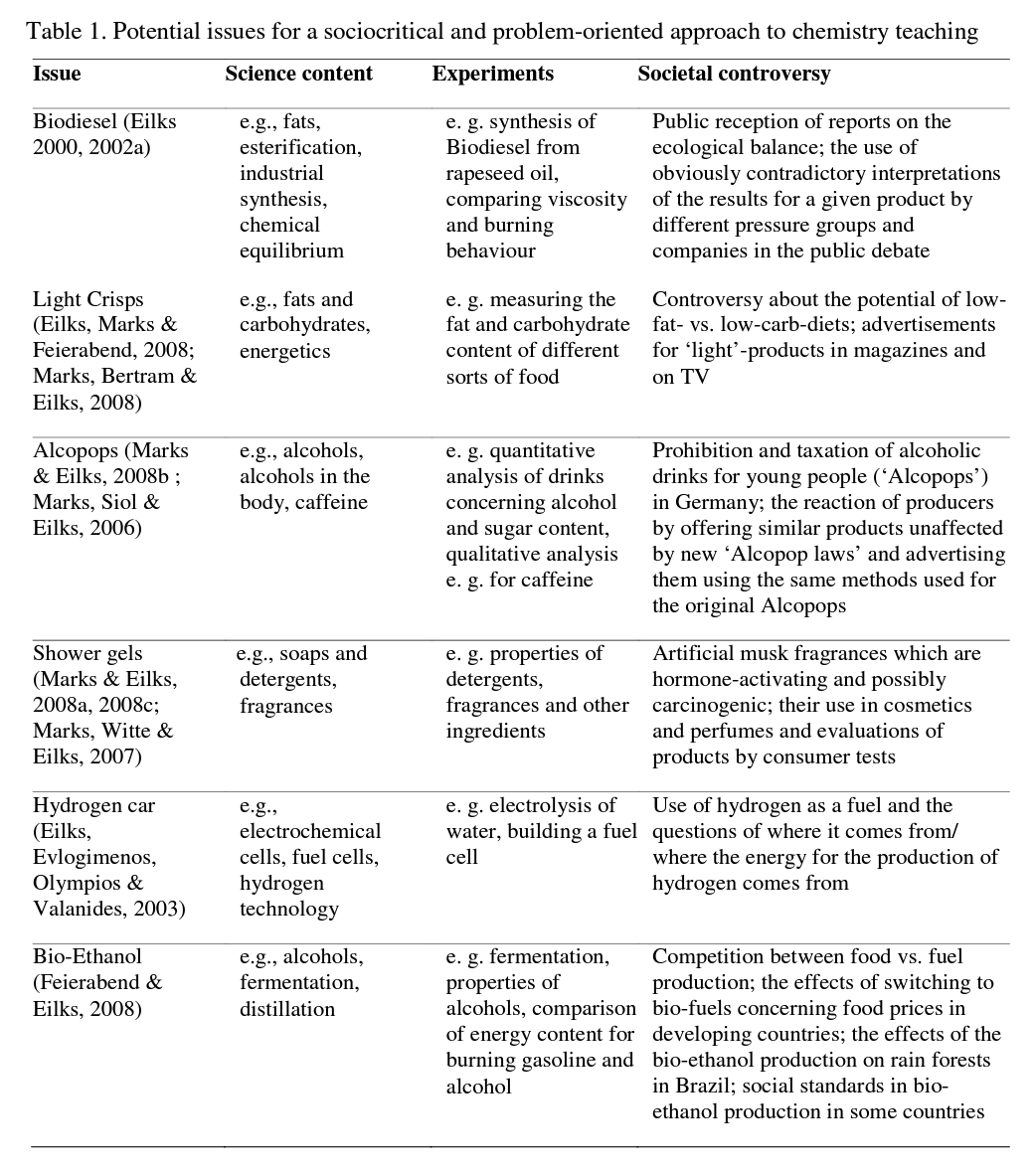
Similar lesson plans have been developed for a variety of issues. All of them start from an authentic and controversial issue within society, which allows for an open discussion. In every case, different fields are touched upon where societal decisions are identified for discussion. Different units, for example, deal with the role of pressure groups in society, journalistic work, the practices of public relations figureheads and advertising experts, or decision-making at the parliamentary level. But all of the issues also reveal questions about an individual's decisions and when they are necessary for using very common products in everyday life. In addition to learning about the controversy normally associated with science in society, all lesson plans embed essential science content learning based on experiments as well as various approaches to co-operative learning. Table 1 gives an overview for some example units.
Experiences, Findings and Discussion, Or: Promoting Scientific Literacy - Or Not?
The development of the teaching approach and the lesson plans presented above took place over eight years in a participatory action research (PAR) project (Bilks, & Ralle, 2002) consisting of a group of teachers from a variety of schools working on different questions of curriculum development and classroom research (Bilks, 2003, 2007). This approach was chosen to sustainably implement STS-oriented teaching in the classroom while simultaneously hindering teachers from automatically falling back into habitual teaching patterns when confronted by new strategies in an in-service course design only (Pedretti & Hodson, 1995; Rannikmäe, 2006). In PAR projects, practicing teachers and university researchers in chemistry education jointly develop lesson plans, teaching methods and materials. From a systematic analysis of different sources of information (i.e., research reports, personal experiences of the teachers, didactical and methodological analysis, or reflections about the chemistry content structure), first-draft lesson plans are negotiated within the action research group. This continues until all practitioners agree that the new lesson plan can potentially help to improve teaching practice. Through subsequent cycles of development, testing, evaluation and reflection/revision, the lesson plans are improved step-by-step. Accompanying the process of development, different kinds of evaluation data are collected as a baseline for better understanding the effects of the lesson plan and the implemented changes. From these studies (e.g., Bilks, 2002a; Bilks, Marks & Feierabend, 2008; Marks & Bilks, 2008a) a huge body of information is available covering teachers’ reflections in the action research group meetings, classroom observations, students’ feedback questionnaires, and different studies based on group discussions.
Some careful conclusions about successfully promoting scientific literacy can be drawn from the various studies based on the sociocritical, problem-oriented approach to chemistry teaching: Teachers and students consistently described the teaching situation as very motivating and intense. It was observed that the intense discussion of socioscientific issues often didn’t stop at the end of the classroom period and often stretched into the students' personal breaks between classes. Students repeatedly mentioned that, for the first time ever, they had perceived school chemistry as being relevant to them and that it was connected to their everyday lives as well as to other school subjects and disciplines. Changes in the attitudes and opinions among some of the students can be found, although analyzing the discussions of students in class and the accompanying group discussions is not easy and is sometimes ambiguous (Albe, 2008). The intense discussions, especially due to their continuation after the lessons were over, indicate that the students accepted all the above-listed topics as interesting and relevant. In open-ended questionnaires, most students overwhelmingly characterized the specific examples as being good starting places from which to teach chemistry (e.g. Marks, Bertram & Bilks, 2008; Marks & Bilks, 2008a). Therefore, the experiences with the different lesson plans support the idea that involving authentic and, especially, controversial debates on socio-scientific issues has the potential to promote students’ interest in science education (Osborne, Driver & Simon, 1998). This includes their skills in communication and evaluation (Holbrook, 1998; Osborne, Erduran & Simon, 2004) and is not only restricted to communication and evaluation within chemistry as a scientific discipline, but also within a framework of understanding chemistry and technology as important parts of our modern world. Some quotes from students’ reflection about the lessons may illustrate this:
We have seen, (...) that all the products have advantages and disadvantages. Of course, in public the interest groups present only the products’ advantages, because that’s positive for themselves, too. They don’t mention the disadvantages. So they are conveniencebased ... we [the public relations experts] want to promote sales of the crisps and do not say any negative things about them. (Issue ‘Light crisps’)
We learned about a critical re-thinking about issues where the answer originally appeared to be so easy. (Issue ‘Bio-Ethanol’)
I learned a lot about shower gels, soaps and their ingredients, which I normally never would have expected. Additionally, I learned that not all ingredients are good for the environment and that you have to look at everything from two perspectives, for example, in the case of musk fragrances ... (Issue ‘Musk fragrances in shower gels’)
I think that it is difficult to have an opinion to this question. On the one hand, there is the ‘danger’ for the environment when synthetic musk fragrances are used. We have to ask ourselves, whether it is more sensible to find a solution to the water purification side of the problem, or to continue research for other ‘healthier’ musk fragrances. On the other hand, natural musks still exist, whereby the problem is that the animals which produce it are threatened by extinction. We can only hope that enough money is dedicated to research efforts. (Issue ‘Musk fragrances in shower gels’)
I learned a lot about the production, structure, use, advantages and disadvantages of biodiesel. Also, I consider it to be important that I learned about our environment and its protection. I especially learned about how companies sell environmentally friendly products and how naive we can be if there is the syllable “bio” in it. (Issue ‘Bio-Diesel’)
I have learned about the advantages and disadvantages of bio-diesel, about interests of pressure groups and how to evaluate their opinions by considering their particular interests, and how to develop an opinion and make up my own mind. (Issue ‘Bio-Diesel’)
From the theoretical side it seems that chemistry topics must include more than contexts (even if they stem from everyday-life) in order to motivate student science learning and stimulate pupils’ interest and critical skill building (Marks, Bertram & Eilks, 2008; Marks & Eilks, 2008b). From our experience we would conclude that topics must be relevant, authentic, and controversial. Controversy in the eyes of the students apparently allows chemistry lessons to focus on the general objectives of education through science. The examples described here, including that of musk fragrances in shower gels and the evaluation of shower gels by consumers or consumer test magazines, seem to offer valuable assistance in this respect. But from the discussions we can also recognize that essential science content learning and understanding is necessary for students to participate in fruitful, substantial discussions (e.g., Lewis & Leach, 2006; Marks, Bertram & Eilks, 2008). Within such topics, students should also have enough room to argue their own opinions. By bringing these aspects together the above examples give some slight indication that students view chemistry lessons differently after such teaching units.
In our view the sociocritical and problem-oriented approach to chemistry teaching seems promising in respect to promoting higher-order cognitive skills, that is, in communication, reflection and evaluating controversial issues within the STS-framework. The students - at least a higher portion of them - seem to profess recognition of a higher relevance of chemistry education to their lives. Within the lessons and in the group discussions from the accompanying research, passages were recorded that support this conclusion. Students appeared to become more self-reflective and openly critical about the way both society and media deal with such debates. From the different studies we can reasonably assume that the approach described can potentially promote the essential skills of well-developed scientific literacy among at least some students when discussing and evaluating controversial issues taken from their everyday lives and society.
References
- Aikenhead, G.S (2007). Humanistic perspectives in the science curricula. In S.K. Abell & N.G. Leder- mann (Eds.), Handbook of research in science education (pp. 881-910). Mahwah, NJ: Lawrence Erlbaum.
- Albe, V. (2008). When scientific knowledge, daily life experience, epistemological and social considerations intersect: Students’ argumentation in group discussions on a socioscientific issue. Research in Science Education, 38(1), 67-90.
- Artola-Garicano, E., Borknent, I., Hermens, J., & Vaes, W. (2003). Removal of two polycyclic musks in sewage treatment plants: Freely dissolved and total concentrations. Environmental Science & Technology, 37(14), 3111-3116.
- Bester, K. (2007). Personal care compounds in the environment: Pathways, fate and methods for determination. Weinheim, Germany: Wiley-VCH.
- Black, P., & Atkin, J.M. (Eds.), (1996). Changing the subject: Innovations in science, mathematics and technology education. London: Routledge/OECD.
- Bybee, R.W. (1987). Science education and the science-technology-society (STS) theme. Science Education, 71(5), 667-683.
- Bybee, R.W. (1997). Toward an understanding of scientific literacy, In W. Gräber & C. Bolte (Eds.), Scientific literacy: An international symposium (pp. 37-68). Kiel, Germany: IPN.
- Driver, R., Leach, J., Millar, R., & Scott, P. (1996). Young people’s image of science. Buckingham, UK: Open University Press.
- Duedahl-Olesen, L., Cederberg, T., Pedersen, K.H., & Hojgard, A. (2005). Synthetic musk fragrances in trout from Danish fish farms and human milk. Chemosphere, 61(3), 422-431.
- Eilks, I. (2000). Promoting scientific and technological literacy: teaching biodiesel. Science Education International, 11(1), 16-21.
- Eilks, I. (2002a). Teaching ‘biodiesel’: A sociocritical and problem-oriented approach to chemistry teaching, and students’ first views on it. Chemistry Education: Research Practice in Europe, 3(1), 67-75.
- Eilks, I. (2002b). ‘Learning at stations’ in secondary level chemistry lessons. Science Education International, 73(1), 11-18.
- Eilks, I. (2003). Cooperative curriculum development in a project of participatory action research within chemical education: Teachers’ reflections. Science Education International, 14(4), 41-49.
- Eilks, I. (2007, August). From technical to emancipatory action research: A six year case study on science teachers involved in a cooperative curriculum development project. Paper presented at the 6th ESERA-Conference, Malmo, Sweden.
- Eilks, I., & Bester, K. (2003). Noch immer geht zu viel Müll den Bach runter": Zur Behandlung von Mülltrennung und Abwasserklärung in der Jahrgangsstufe 7 [Trans. Still too much waste runs down the river: On the teaching about waste treatment and waste water clarification in grade 7]. Praxis der Naturwissenschaften - Chemie in der Schule, 52(8), 37-43.
- Eilks, I., Evlogimenos, S., Olympios, C., & Valanides, N. (2003). Ecologically friendly cars, without emissions. Should they be compulsory? In J. Holbrook (Ed.), A resource book for teachers in science subjects UNESCO/ICASE 2003. Retrieved 01 October, 2008, from http://www.lmnt.org/lmntnytt/Fuel%20Cell2.pdf
- Eilks, I., Marks, R., & Feierabend, T. (2008). Science education research to prepare future citizens: Chemistry learning in a sociocritical and problem-oriented approach. In B. Ralle & I. Eilks (Eds.), Promoting successful science learning : The worth of science education research (pp. 75- 86). Aachen, Germany: Shaker.
- Eilks, I., & Ralle, В. (2002). Participatory action research in chemical education. In B. Ralle & I. Eilks (Eds.), Research in chemical education: What does this mean? (pp. 87-98). Aachen, Germany: Shaker.
- Elmose, S., & Roth, W.-M. (2005). Allgemeinbildung: Readiness for living in a risk society. Journal of Curriculum Studies, 37(1), 11-34.
- Eschke H.D., Dibowski, H.J., & Traud, J. (1995a). Untersuchungen zum Vorkommen polycyclischer Moschus-Duftstoffen in verschiedenen Umweltkompartimenten, 2. Mitteilung [Trans. Studies on the occurrence of polycyclic musk flavors in different environmental compartments. 2nd Communication]. UWSF Zeitschrift fur Umweltchemie und Ökotoxikologie, 7(3), 131-138.
- Eschke H.D., Dibowski, H.J., &Traud, J. (1995b). Nachweis und Quantifizierung von polycyclischen Moschus-Duftstoffen mittels Ion-Trap GC/MS/MS in Humanfett und Muttermilch [Trans,\. Detection and quantitative analysis of musk fragrances by means of ion-trap GC/MS/MS in human fat and breast milk]. Deutsche Lebensmittel-Rundschau, 97(12), 375-379.
- Feierabend, T., & Eilks, I. (2008). Bioethanol: Bewertungs- und Kommunikationskompetenz schulen in einem gesellschaftskritisch-problemorientierten Chemieunterricht [Trans. Bio-ethanol: Promoting competencies in evaluation and communication within the sociocritical and problem- oriented approach to chemistry lessons]. Der Mathematische und Naturwissenschaftliche Unterricht, 62(2), 92-97.
- Fensham, P. (2004). Increasing the relevance of science and technology education for all students in the 21st century. Science Education International, 75(1), 7-27.
- Fischer, H. E., Klemm, K., Leutner, D., Sumfleth, E., Tiemann, R., & Wirth, J. (2005). Framework for empirical research on science teaching and learning. Journal of Science Teacher Education, 76(4), 309-349.
- Gaterman, R., Biselli, S., Hühnerfuss, H., Rimkus, G. G., Hecker, M., & Karbe, L. (2002). Synthetic musks in the environment. Part 1: Species-dependant bioaccumulation of polycyclic and nitro musk fragrances in freshwater fish and mussels. Archives of Environmental Contamination and Toxicology, 42(4), 437-446.
- Gräber, W. (2002). Chemistry education’s contribution to scientific literacy: An example. In B. Ralle & I. Eilks (Eds.), Research in chemical education: What does this mean? (pp. 119-128). Aachen, Germany: Shaker.
- Hajslova, J., Gregor, P., Chadlova, V., & Alterova, K. (1998). Musk compounds in fish from Elbe River. Organohalogen Compounds, 39,253-256.
- Holbrook, J. (1998). Operationalising scientific and technological literacy - a new approach to science teaching. Science Education International, 9(2), 13-18.
- Holbrook, J. (2003). Increasing relevance of science education: The way forward. Science Education International, 14(1), 5-13.
- Holbrook, J., & Rannikmäe, M. (2007). The nature of science education for enhancing scientific literacy. International Journal of Science Education, 29(11), 1347-1362.
- Klafki, W. (2000). The significance of classical theories of Bildung for a contemorary concept of Allgemeinbildung. In I. Westbury, S. Hopmann & K. Riquarts (Eds.), Teaching as a reflective practice'. The German Didaktik tradition (pp. 85-107). Mahwah,NJ: Lawrence Erlbaum.
- Lee, M.-К., & Erdogan, I. (2007). The effect of science-technology-society teaching on students’ attitudes toward science and certain aspects of creativity. International Journal of Science Education, 29(3), 1315-1328.
- Lewis, J., & Leach, J. (2006). Discussion of socioscientific issues: the role of science knowledge. International Journal of Science Education, 28(11), 1267-1287.
- Marks, R., Bertram, S., & Eilks, I. (2008). Learning chemistry and beyond with a lesson plan on potato crisps, which follows a socio-critical and problem-oriented approach to chemistry lessons: A case study. Chemistry Education: Research and Practice, 9(3), 267-276.
- Marks, R., & Eilks. I. (2008a). Nachrichtenspots erstellen über Chemie - Ein Weg Kommunikationsund Bewertungskompetenz zu schulen? [Trans. Writing news spots about chemistry: A way to teach communication and evaluation competencies?]. Der Mathematische und Naturwissenschaftliche Unterricht, 67(4), 224-229.
- Marks, R., & Eilks. I. (2008b). Kommunikations- und Bewertungskompetenz entwickeln in einem gesellschaftskritisch-problemorientierten Chemieunterricht über Alcopops - Eine Reflektion aus einem Projekt Partizipativer Aktionsforschung [Trans. Developing communication and evaluation competencies in a sociocritical and problem-oriented approach to chemistry teaching on Alcopops: A reflection from a project of participatory action research]. Chimica etc. Didacticae, 34(101), 48-77.
- Marks, R., Siol, A., & Eilks, I. (2006). Ein Schülerlabor zu Alcopops - eingebettet in einem gesellschaftskritisch-problemorientierten Chemieunterricht [Trans. A students’ lab on Alcopops: Embedded into the sociocritical and problem-oriented approach to chemistry lessons]. Praxis der Naturwissenschaften: Chemie in der Schule, 55(2), 39-47.
- Marks, R., Witte, N., & Eilks, I. (2007). „Riecht gut, aber ..." - Chemie und Bewerten Lernen am Beispiel des Duschgels [Trans. “Smells well, but...”: Learning chemistry and to evaluate on the example of a shower gel]. Praxis der Naturwissenschaften: Chemie in der Schule, 56(3), 11-15.
- Millar, R. (1996). Towards a science curriculum for public understanding. School Science Review, 77(280), 7-18.
- Millar, R. (2006). Twenty-first century science: Insights from the design and implementation of a scientific literacy approach in school science. International Journal of Science Education, 28(13), 1499-1521.
- Morell, P.D., & Lederman. N.G. (1998). Students’ attitudes toward school and classroom science: Are they independent phenomena? School Science and Mathematics, 98(2), 76-83.
- Müller, S., Schmid, P., & Shlatter, C. (1996). Occurrence of nitro and non-nitrobenzenoid musk compounds in human adipose tissue. Chemosphere, 33(1), 17-28.
- Ökotest (Hrsg.). (2004). Duschgele - Nicht ganz sauber [Trans. Shower-gels - not fully clean], ÖKOTEST-Jahrbuch für 2004.
- Osborne J.F. (2001). Science education for contemporary society: Problems, issues and dilemmas. In O. de Jong, E.R. Savelsbergh, & A. Alblas (Eds.), Teaching for scientific literacy (pp. 15-26). Utrecht: CDB.
- Osborne, J.F. (2003). Attitude towards science: a review of the literature and its implications. International Journal of Science Education, 25(9), 1049-1079.
- Osborne, J.F. (2007). Science education for the twenty first century. Eurasia Journal of Mathematics, Science & Technology Education, 3(3), 173-184.
- Osborne, J.F., Driver, R., & Simon, S. (1998). Attitudes to science: issues and concerns. School Science Review, 79(288), 27-33.
- Osborne, J.F., Erduran, S., & Simon, S. (2004). Enhancing the quality of argumentation in school science. Journal of Research Science Teaching, 47(10), 994-1020.
- Pedretti, E., & Hodson, D. (1995). From rhetoric to action: implementing STS education through action research. Journal of Research Science Teaching, 32(5), 463-485.
- Rannikmäe, M. (2006). Using socially derived teaching approaches in science classes: A longitudinal study on teachers learning in a research based curriculum innovation. In I. Eilks & B. Ralle (Eds.), Towards research-based science teacher education (pp. 137-146). Aachen, Germany: Shaker.
- Ratcliffe, M. (1998). Discussing socioscientific issues in science lessons: Pupils’ action and the teacher’s role. School Science Review, 79(288). 55-59.
- Roberts, D.A. (2007). Scientific literacy/science literacy. In S.K. Abell & N.G. Lederman (Eds), Handbook of research in science education (pp. 729-780). Mahwah, NJ: Lawrence Erlbaum
- Roth, W.-M., & Lee S. (2004). Science education as/for participation in the community. Science Education, 88(2), 263-291.
- Sadler, T.D. (2004). Informal reasoning regarding socioscientific issues: A critical review of research. Journal of Research in Science Teaching, 41(5), 513-536.
- Seinen, W., Lemmen, J., Pieters, R., Verbruggen, E. & van der Burg, B. (1999). AHTN and HHB show weak estrogenic - but no uterotrophic activity. Toxicology letters, 111(1-2), 161-168.
- Simonich, S., Federte, T., Eckhoff, W., Rottiers, A., Webb, S., Sabaliunas, D. & de Wolf, W. (2002). Removal of fragrance materials during US and European wastewater treatment. Environmental Science & Technology, 36(13), 2839-2847.
- Simpson, R.D., Koballa, T.R., Oliver, J.S., & Crawley, F.E. (1994). Research on the affective dimension of science learning. In D. L. Gabel (Ed.), Handbook of research in science teaching and learning (pp. 211-234). New York: MacMillan.
- Solomon, J., & Aikenhead, G. (Eds.) (1994). STS education: International perspectives on reform. New York: Teachers College Press.
- Stanat P., Artelt, C., Baumert, J., Rheme, E., Neubrand, M., Prenzel, M., Schiefele, U., Schneider, W., Schümer, G., Tillmann, K.-J., & Weiß, M. (2002). PISA 2000: Overview of the study. Retrieved 01 October, 2008, fromwww.mpib-berlin.mpg.de/pisa/PISA-2000_Overview.pdf
- Van Aalsvoort, J. (2004a). Logical positivism as a tool to analyse problem of chemistry’s lack of relevance in secondary school chemistry education. International Journal of Science Education, 26(9), 1151-1168.
- Van Aalsvoort, J. (2004b). Activity theory as a tool to address the problem of chemistry’s lack of relevance in secondary school chemistry education. International Journal of Science Education, 26(13), 1635-1651.





