The costunolide biosynthesis enzymes of Artemisia glabella Kar. et Kir.: Determination of the nucleotide sequences of the mRNA


Опубликована Апрель 30, 2021
Последнее обновление статьи Авг. 21, 2022
Abstract
Artemisia glabella Kar. et Kir. is a source of sesquiterpene lactone arglabin, which has antitumor, radiosensitizing and immunomodulatory activity. Studying the biosynthesis of arglabin and its derivatives will allow us to develop the biotechnological basis for its production, thereby increasing its availability. The precursor to most sesquiterpene lactones is costunolide. The purpose of these studies was to detect and determine the nucleotide sequences of mRNA enzymes germacrene A synthase (GAS, EC 4.2.3.23), germacrene A oxidase (GAO, EC 1.14.14.95) and costunolide synthase (COS, EC 1.14.13.120), involved in the biosynthesis of costunolide in A. glabella. As a result of studies, mRNA was isolated from various forms (intact plant, regenerant plant, callus) of A. glabella. Using specific primers, mRNA fragments of genes encoding sesquiterpene lactones biosynthesis enzymes were amplified and their nucleotide sequences were determined. A comparative analysis of the obtained sequences showed their high (>90%) identity with the genes of GAS, GAO and COS of other representatives of the family Asteraceae. It was revealed that enzymes are expressed both in an intact plant and in calluses and regenerant plants obtained in vitro.
Ключевые слова
Artemisia glabella Kar. et Kir., costunolide synthase., mRNA, germacrene A synthase, germacrene A oxidase, cDNA, sequencing
INTRODUCTION
MATERIALS AND METHODS
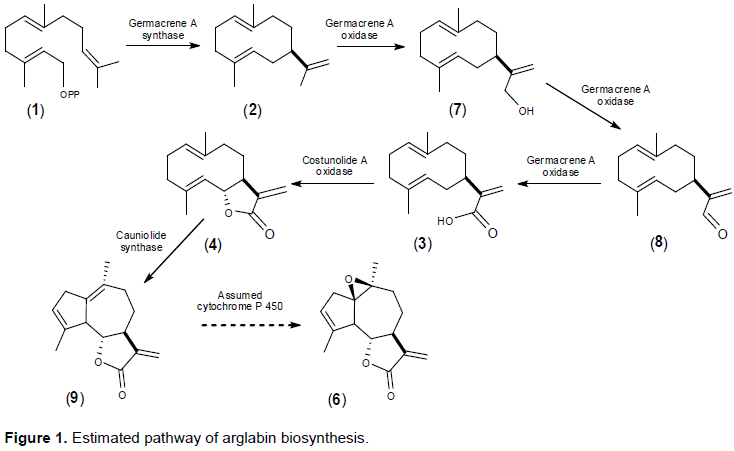
RESULTS AND DISCUSSION
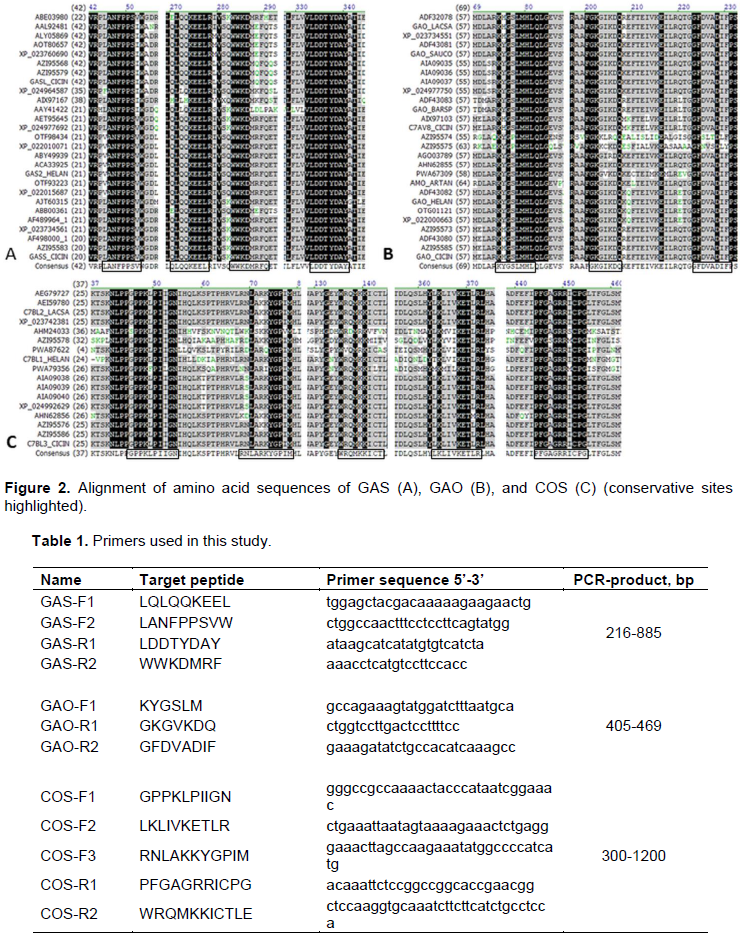
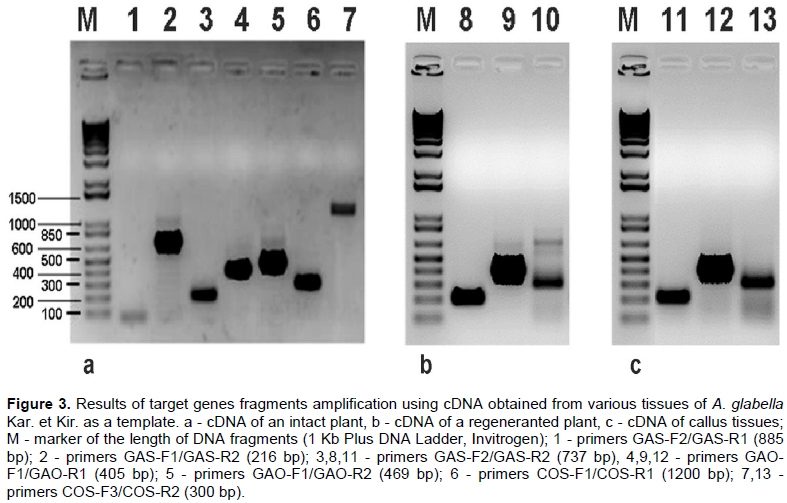
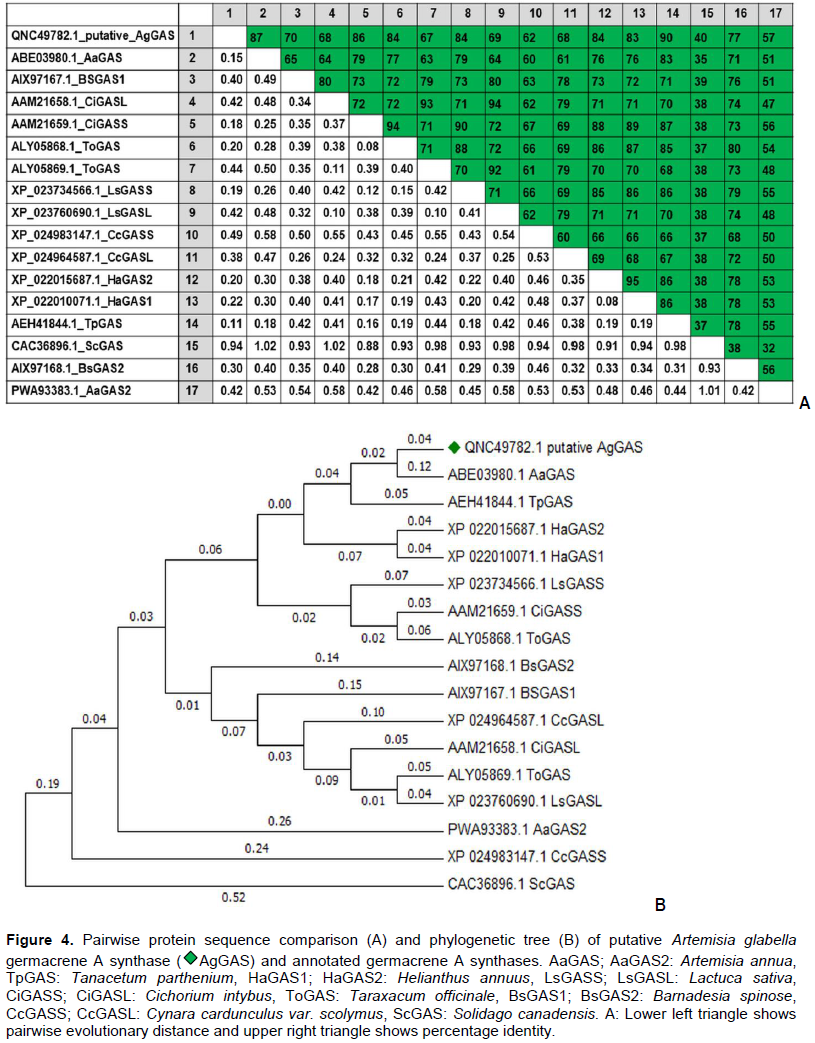
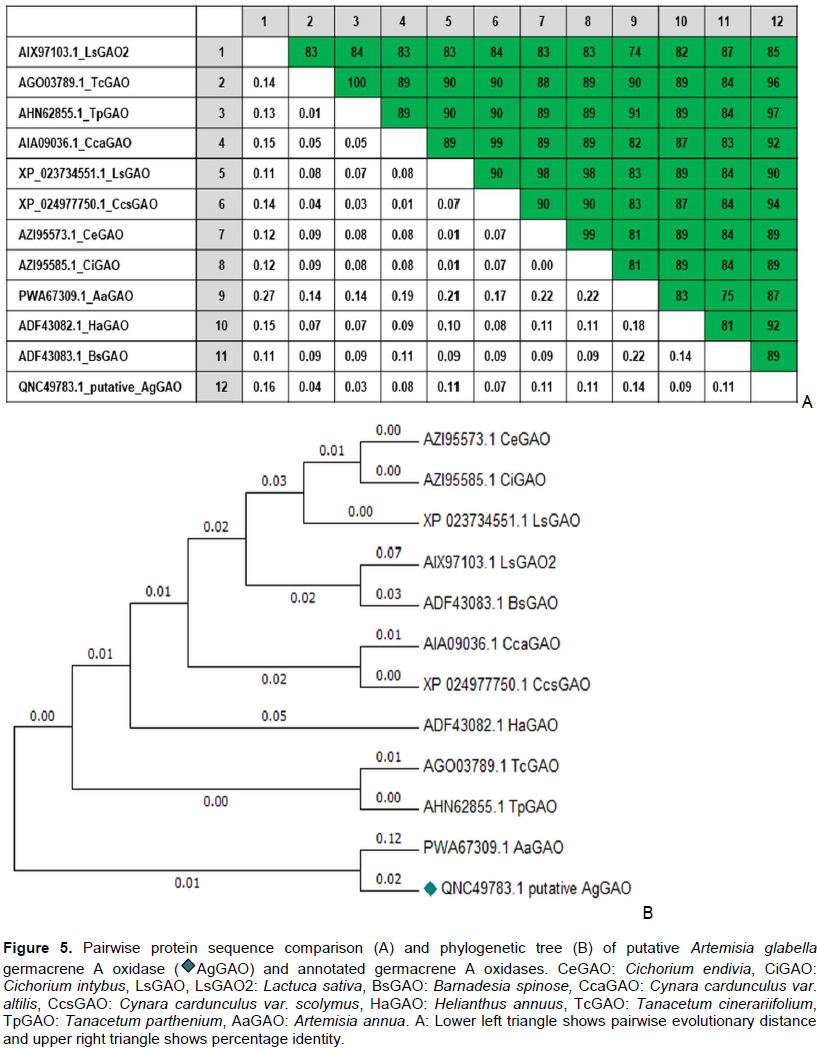
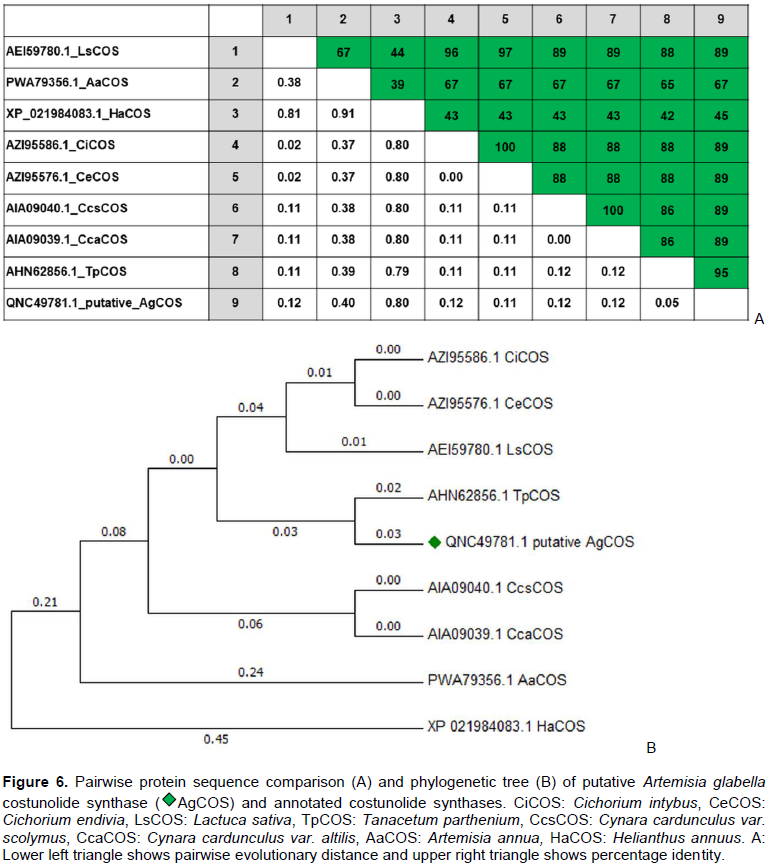
CONCLUSION
CONFLICT OF INTERESTS
ACKNOWLEDGEMENTS
REFERENCES
Adekenov SM (2001). Method and device for production of lyophilized hydrochloride-1β, 10β-epoxy-13-dimethylaminoguaia-3(4)-en-6,12-olide. US6242617B1. | |
Adekenov SM (2015). Method for production of hydrochloride 1(10) beta-epoxy-13-dimethylamino-5,7-alpha,6,11-beta (h)-guaia-3(4)-en-6,12-olide, the lyophilized antitumor preparation "arglabin". EP2069357B1. | |
Adekenov SM, Bouwmeester HJ (2015). About the Biosynthesis of Sesquiterpene Lactone Arglabin. In: Achievements and Prospects for the Development of Phytochemistry, 27-28. Karaganda, Kazakhstan. | |
Adekenov SM, Mukhametzhanov MN, Kagarlitsky AD, Kupriyanov AN (1982). Arglabin - A new sesquiterpene lactone from Artemisia glabella. Chemistry of Natural Compounds 18(5):623-624. | |
Adekenov SM, Rakhimova BB, Dzhazin KA, Alikov VB, Shaushekov ZK, Toregozhina ZhR, Turdybekov KM (1995). Sesquiterpene lactones from Artemisia glabella. Fitoterapia 66(2):142-146. | |
Bouwmeester HJ, Kodde J, Verstappen FWA, Altug IG, de Kraker J-W, Wallaart TE (2002). Isolation and Characterization of Two Germacrene A Synthase cDNA Clones from Chicory. Plant Physiology 129(1):134-144. | |
de Kraker J-W, Franssen MCR, Joerink M, de Groot A, Bouwmeester HJ (2002). Biosynthesis of Costunolide, Dihydrocostunolide, and Leucodin. Demonstration of Cytochrome P450-Catalyzed Formation of the Lactone Ring Present in Sesquiterpene Lactones of Chicory. Plant Physiology 129(1):257-268. | |
Fischer NH, Czerson H, Bohlmann F, Stuessy TF (1979). Sesquiterpenoid and acetylenic constituents of seven Clibadium species. Phytochemistry 18(2):257-260. | |
Frederick CS (1982). Sesquiterpene lactones as taxonomic characters in the asteraceae. The Botanical Review 48(2):121-594. | |
Ikezawa N, Göpfert JC, Nguyen DT, Kim SU, O'Maille PE, Spring O, Ro DK (2011). Lettuce costunolide synthase (CYP71BL2) and its homolog (CYP71BL1) from sunflower catalyze distinct regio- and stereoselective hydroxylations in sesquiterpene lactone metabolism. The Journal of biological chemistry 286(24):21601-21611. | |
Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870-1874. | |
Liu Q, Kashkooli AB, Manzano D, Pateraki I, Richard L, Kolkman P, Lucas MF, Guallar V, Ric CH de Vos, Franssen MCR., Van der Krol A, Bouwmeester H (2018). Kauniolide synthase is a P450 with unusual hydroxylation and cyclization-elimination activity. Nature Communications 9(1):1-13. | |
Ma GH, Chen KX, Zhang LQ (2019). Advance in biological activities of natural guaiane-type sesquiterpenes. Medicinal Chemistry Research 28((9):1339-1358. | |
Majdi ?, Liu Q, Karimzadeh G, Malboobi M, Beekwilder J, Cankar K, Todorovic S, Simonovic A, Bouwmeester HJ (2011). Biosynthesis and localization of parthenolide in glandular trichomes off ever few (Tanacetum parthenium L. Schulz Bip.). Phytochemistry 72(14-15):1739-1750. | |
Mantler SN, Zhakanov MM, Adekenov SM (2020). Biosynthesis and dynamics of accumulation of sesquiterpene lactones in Artemisia glabella Kar. et Kir. News of the national academy of sciences of the republic of Kazakhstan - Series chemistry and technology 4(442):22-29. | |
Menin B, Comino C, Portis E, Moglia A, Cankar K, Bouwmeester HJ, Lanteri S, Beekwilder J (2012). Genetic mapping and characterization of the globe artichoke (+)-germacrene A synthase gene, encoding the first dedicated enzyme for biosynthesis of the bitter sesquiterpene lactone cynaropicrin. Plant science: an international journal of experimental plant biology 190:1-8. | |
Nguyen TD, Faraldos JA, Vardakou M, Salmon M, O'Maille PE, Ro DK (2016). Discovery of germacrene A synthases in Barnadesia spinosa: The first committed step in sesquiterpene lactone biosynthesis in the basal member of the Asteraceae. Biochemical and biophysical research communications 479(4):622-627. | |
Nguyen TD, Kwon M, Kim SU, Fischer C, Ro DK (2019). Catalytic Plasticity of Germacrene A Oxidase Underlies Sesquiterpene Lactone Diversification. Plant physiology 181(3):945-960. | |
Pazouki L, Memari HR, Kännaste A, Bichele R, Niinemets Ü (2015). Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: gene cloning, functional characterization and expression analysis. Frontiers in plant science 6:111. | |
Saitou N, Nei M (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4(4):406-425. | |
Xu H, Dickschat JS (2020). Germacrene A - A Central Intermediate in Sesquiterpene Biosynthesis. Chemistry (Weinheim an der Bergstrasse, Germany) 26(72):17318-17341. | |
Zuckerkandl E, Pauling L (1965). Evolutionary divergence and convergence in proteins. In: Evolving Genes and Proteins. Ed. by Bryson V and Vogel HJ, Academic Press pp. 97-166, New York. | |





