The significant inputs of trace elements and rare earth elements from melting glaciers in Antarctic coastal waters


Опубликована Май 7, 2015
Последнее обновление статьи Авг. 20, 2023
Abstract
To evaluate the impact of modern glacier melting on the chemical enrichment of Antarctic coastal waters, we measured trace elements, including dissolved iron (Fe) and rare earth elements (REEs), together with dissolved inorganic nitrogen, phosphorous, silicate and dissolved organic carbon (DOC) in ice, snow and coastal seawater of Marian Cove in the northernmost part of Antarctica (62°S). There was an increase in the concentrations of Fe and other trace elements (Al, Mn, Cr, Ni, Co, Pb and REEs) between the bay mouth and the glacier valleys. Good correlations between salinity and these chemical elements indicate that the trend was mainly due to the influence of glacier meltwater (GMW). When the effect of GMW was quantified based on plots of its presence (average 5.7%) in the surface water of the cove, the concentrations of trace elements in seawater increased 18-fold for Fe, eight- to 10-fold for Al and Mn and up to four-fold for Cr, Ni, Co, Pb and REEs by GMW. However, the contribution of GMW to inorganic nutrients and DOC was negligible. The significance of GMW-borne REE contribution in this cove was further evidenced by middle REE enrichment in cove water. Our results suggest that the currently increasing glacier melting in Antarctica has a significant influence on the level of trace elements, particularly Fe, in cove water, which in turn may have a significant impact on the biogeochemistry of coastal seawater in Antarctica.
Ключевые слова
Antarctica, trace elements, Marian Cove, glacier melting, rare earth elements, Iron
At the latest count, there were approximately 200 000 icebergs in the Southern Ocean, with linear dimensions between 50 m and tens of kilometres (Williams et al. 1999; Smith et al. 2007). Depending on their size, some icebergs are grounded whereas others can drift over hundreds to thousands of kilometres within several weeks (Schodlok et al. 2006; Schwarz & Schodlok 2009; Luckman et al. 2010). Antarctic glaciers have retreated in recent years, which has resulted in a loss of mass from ice shelves (Scambos et al. 2000; Bindschadler & Rignot 2001; Long et al. 2002; Rignot & Steffen 2008; Rignot et al. 2013). The disintegration of Antarctic ice shelves over the past 50 years has been attributed to several processes: atmospheric warming (Cook & Vaughan 2009), an increase in basal melt of glaciers by warm waters (Jacobs et al. 2011; Pritchard et al. 2012) and changes in coastal polynyas (Khazendar et al. 2013). Such processes have contributed to an increase in the numbers of icebergs in the Southern Ocean (Rignot & Thomas 2002; Velicogna & Wahr 2006; Rignot et al. 2011).
Glaciers acquire a significant load of terrigenous material through glacial processes, dust accumulation and direct contact with shelf sediments. Glacier fragments (or ice fragments) containing elevated levels of land-borne material are released directly into ambient waters by melting (Maher & Dennis 2001; Raiswell et al. 2008). The increase in iceberg calving from Antarctica over the last few decades has therefore provided new opportunities to evaluate the significance of terrestrial micronutrient inputs into the Southern Ocean (Smith et al. 2007; Shaw et al. 2011; Alderkamp et al. 2012). For example, glaciers have been suggested as an important source of iron (Fe) to the surrounding waters (de Baar et al. 1995; Raiswell et al. 2008; Raiswell 2011). Amongst terrigenous elements, Fe is particularly important in the Southern Ocean because it can limit primary productivity (Martin 1990; Buma et al. 1991; Sunda & Huntsman 1997; Coale et al. 2003).
Recent studies have shown the potential importance of glacier melting to the supply of new Fe and the subsequent effect on marine production in the Southern Ocean (Statham et al. 2008; Schwarz & Schodlok 2009; Lin et al. 2011; Alderkamp et al. 2012). Shaw et al. (2011) reported that free drifting icebergs are important for terrigenous material inputs to surface waters in the Southern Ocean. Gerringa et al. (2012) also suggested that large Fe inputs from Pine Island glaciers by the lateral advection could supply the total Fe demand by a phytoplankton bloom. More recently, Bhatia et al. (2013) showed that the annual flux of bioavailable Fe from Greenland ice meltwater was comparable to that of dust-derived soluble Fe inputs to the North Atlantic. However, little is known about the role of melting glaciers on the distribution of trace elements in the surrounding seawater because their sources are diverse and the interactions with the ecosystem are highly dynamic.
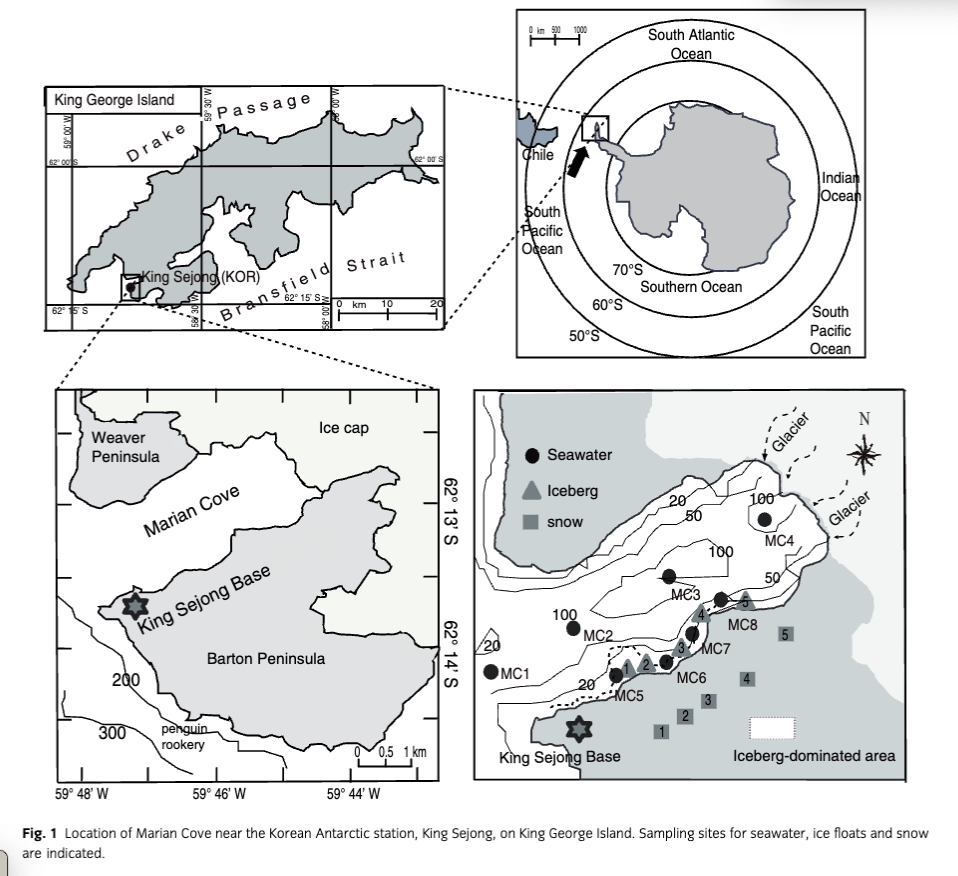
The main objectives of this study are (1) to determine the distribution of dissolved trace elements including Fe and rare earth elements (REEs), dissolved inorganic nitrogen (DIN), phosphorous (DIP), silicate (DSi) and dissolved organic carbon (DOC) in ice floats, snow and coastal seawater and (2) to quantify trace elements and chemical components derived from Antarctic glacier melts in a coastal embayment. This study was conducted at Marian Cove, which is located south of the Antarctic Polar Front (Fig. 1). It was selected as an ideal place to study the impacts of glacial melt on coastal waters because glacier valleys drain into Marian Cove and deliver large volumes of turbid glacier meltwater (GMW) during the austral summer (Shim et al. 2011).
Materials and methods
Study area
This study was conducted at Marian Cove near the King Sejong research base (Korean Antarctic station; 62°S, 59.7°W) from 21 to 28 January 2012 (Fig. 1). The base is located on the Barton Peninsula on King George Island, the largest island in the South Shetland Archipelago (Fig. 1). Marian Cove (3.5 km long and 1.2 km wide) is a small fjord with a maximum depth of about 100 m and is located in an inlet of Maxwell Bay, south of the Antarctic Polar Front (Yoo et al. 2000). Surface temperatures and salinities in the cove range from −2 to 2°C (average −0.4°C) and from 32 to 35 (average 33.8), respectively (Shim et al. 2011).
Marian Cove is free of sea ice for most of the year, but thick annual sea ice appears during winter. During the austral summer, the cove is completely ice-free (from November to February), which leads to enhanced biological productivity (Klöser et al. 1993; Ahn et al. 1997). Small valley glaciers draining into Marian Cove deliver icebergs and large volumes of turbid meltwater during the summer months (Yoon et al. 1998). Most ice-rafted debris originates from icebergs calved off the edges of glaciers, whereas fine-grained particles are discharged by glacier melt during the summer (Shim et al. 2011). Substantial amounts of snow meltwater are also discharged into the bay during this period, although the volume attributed to this type of meltwater has not yet been determined.
Sampling
Surface seawater samples for dissolved trace elements analysis was collected from the coastline or on board a Zodiac boat using a handle sampler (1 L polyethylene dipper with a 4 m handle length). The dippers were soaked and pre-cleaned with double-distilled 1 M ultra-pure HNO3 (65%, Thermo Fisher Scientific, Waltham, MA, USA) for at least 24 h before sampling. Salinities were measured in situ using an YSI 30 portable conductivity sensor (YSI, Yellow Springs, OH, USA) and an Ocean Seven 304 conductivity–temperature–depth logger (Idronaut, Brugherlo, Italy).
Ice samples that had drifted to the coast after calving events were manually collected from the near-shore region and bagged in clean plastic zippered bags. Back in the laboratory, the ice samples were rinsed briefly with distilled water to remove any possible surface contamination, and the rinse water was left to drip from the ice for a few minutes. Finally, the ice samples were transferred to new clean zippered bags and melted completely for trace element analyses. Old glacier ice and recently formed ice were included in the ice samples, but no sea ice was present. Snow samples consisting of a mixture of recently formed and old glacier ice were collected in acid-cleaned high-density polyethylene (HDPE) bottles from land sites near King Sejong Base (Fig. 1) and transported to the clean laboratory bench.
All samples were collected in HDPE bottles (1 L) that had been pre-cleaned with 1 M ultra-pure HNO3 and then rinsed with de-ionized water (DIW). The samples were double-bagged with clean plastic zippered bags and moved to the clean bench at King Sejong Base where they were passed through a pre-cleaned membrane filter (0.45 µm, mixed cellulose ester) and then acidified with 6 M ultra-pure HNO3 (15.8 M, Thermo Fisher Scientific) to 0.03 M of HNO3 (pH<1.5) within 5 h of initial sampling. The samples were then stored in a freezer for later analyses.
Pre-concentration of trace elements
In this study, we define dissolved trace elements as the total amount of trace elements extracted by a Chelex 100 column (Bio-Rad, Hercules, CA, USA). Colloidal and nanoparticulate phases retained on the column were also included in the measurements. These phases were not retained by the physical filtration process as they are much smaller than the pore size of the packed column (10 µm), and it was therefore considered that they were retained by a surface reaction or binding. The concentrations of dissolved trace elements (including Fe) in water samples were measured using the Chelex 100 resin column method developed by Lewis & Landing (1991), Öztürk (1995), Öztürk et al. (2003) and Ardelan et al. (2010), and recently improved by Kim & Kim (2011) and Jeong et al. (2012). Briefly, the aqueous samples (100–500 mL) were adjusted to pH 5.7–5.9 using pure CH3COOH (99.7%, Junsei Chemical Co., Tokyo, Japan) and NH3 (28%, Junsei). Then, the samples were passed through a Chelex 100 resin (1.2 g, wet weight) column at a flow rate of less than 1.2 mL min−1. The Chelex 100 resin was pre-cleaned with 2 M HNO3 followed by DIW (resistivity >18.2 MΩ) (Human Power, Seoul, Korea) and then soaked in 1 M ammonium acetate overnight before being packed into the column, which effectively buffered the resin at pH 6. The size of the column used in this study was 85 mm×8 mm (Eichrom Technologies, Lisle, IL, USA). Eight millilitre of the 1 M ammonium acetate solution (Sigma Aldrich, St. Louis, MO, USA) and 5 mL of DIW were used to wash out the interfering elements, and the trace elements absorbed onto the resin were then eluted with 1.5–2.0 mL of 2 M HNO3. All procedures for the extraction of trace elements from samples were conducted at a clean bench (class-100) installed at King Sejong Base.
The determination of trace elements using inductively coupled plasma mass spectrometry
Dissolved trace elements were measured by inductively coupled plasma mass spectrometry (model X-II, Thermo Scientific Waltham, MA, USA). The standards used for trace elements and REEs were diluted from standard stock solutions (SCP Science, Quebec, Canada). The procedural blank values (n=9) were 240±150, 3±1, 12±2, 26±4, 5±1, 12±8 and 16±3 pM for Al, Cr, Mn, Ni, Cu, Zn and Pb, respectively. The procedural blank values of Co and Cd were lower than the detection limits (<1 pM). Our total procedural blank values were obtained from full volume (500 mL) samples of artificial seawater for all steps of pre-concentration and analysis. The blank concentrations are therefore for pre-concentrated (about 250-fold) solutions. All blank values were lower than 5% of the average seawater concentrations, with the highest value for Fe (about 5%).
The recovery test for this column extraction method was conducted by spiking approximately 100 times the average seawater concentrations of trace elements to the seawater samples. When these spiked samples were pre-concentrated, the recoveries of Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb were 103±11, 92±6, 97±15, 87±17, 93±10, 88±16, 75±17, 108±31, 118±34 and 110±9% (n=3 for trace metals and n=9 for Fe), respectively. The recovery efficiencies for seawater samples were higher than 90% for most of these trace elements. All the concentrations in this study were corrected by this recovery factor. To check the validity of this method, we also analysed common seawater reference samples (CASS-4 and NASS-5, National Research Council of Canada) for trace elements (Table 1).
Table 1 Measured concentrations of trace elements for the certified material (CASS-4 and NASS-5) (±1σ, n=3). | ||||
| Certified reference material (unit: µg/L) | |||
| CASS-4 | NASS-5 | ||
Element | Reference value | This study | Reference value | This study |
Cr | 0.14±0.02 | 0.09±0.05 | 0.11±0.02 | 0.03±0.03 |
Mn | 2.78±0.19 | 2.91±0.07 | 0.92±0.06 | 1.24±0.16 |
Fe | 0.71±0.06 | 0.50±0.10 | 0.30±0.04 | 0.65±0.10 |
Co | 0.03±0.00 | 0.03±0.01 | 0.01±0.00 | 0.01±0.01 |
Ni | 0.31±0.03 | 0.34±0.08 | 0.25±0.03 | 0.27±0.07 |
Cu | 0.59±0.06 | 0.70±0.03 | 0.30±0.04 | 0.50±0.13 |
Zn | 0.38±0.06 | 0.21±0.25 | 0.10±0.04 | 0.05±0.2 |
Cd | 0.03±0.00 | 0.02±0.02 | 0.02±0.00 | 0.02±0.01 |
Pb | 0.01±0.00 | 0.02±0.04 | 0.01±0.01 | 0.01±0.00 |
The validity of this method for Fe was tested more carefully. The Chelex 100 blank for Fe was <0.36 nM, which falls in the range of blanks from other studies (0.2±0.08 nM Fe) using the same resin (Öztürk 1995; Ardelan et al. 2010). The levels of Fe for the reagent blanks (ultra-pure HNO3, CH3COOH, NH3 and ammonium acetate solution, n=18) and procedural blanks (n=25) were 0.1±0.04 nM and 0.3±0.1 nM, respectively. Our total blank level of Fe was similar to the values from other recent studies (0.1–0.25 nM) that used different analytical methods (Ardelan et al. 2010; Milne et al. 2010; Lee et al. 2011). The detection limit of Fe was 0.14 nM, which was three times the standard deviation of the instrumental blanks.
The procedural blank values for La, Ce, Nd, Tb, Tm and Lu (i.e., REEs) were 4.5, 1.4, 1.3, 0.4, 0.7 and 0.5 pM, respectively, whereas values for the other REEs were not detectable. The instrumental detection limits of La and Gd were 4.0 and 2.7 pM, respectively, while those of the other elements were <2 pM. The extraction efficiencies for REEs were >95%. The relative standard deviation (RSD%) of each REE measurements was lower than 2, 3 and 5% for the light, middle and heavy REE (LREE, MREE and HREE), respectively. The BaO/Eu and CeO/Ce ratios were found to be lower than 2 and 1%, respectively, when 1 ppb of REEs was measured before the sample measurement. Although the Chelex resin extraction method eliminates most of the major alkaline and alkaline earth metals (Na+, Mg2+, K+, and Ca+), up to 75 nM of Ba concentrations remained in the extracted samples. Because high amounts of Ba and its oxides, such as BaO or BaOH+, can lead to polyatomical interferences to Eu, 151Eu and 153Eu were monitored using Ba solutions of 15–70 nM, which are similar to the range observed in the eluted solution of seawater. In all trials (n=50), no Eu was detected, except for in a few cases where <3 pM Eu (<4% of the Eu concentrations in seawater samples) was detected. In addition, no increase in Eu concentrations was observed when 2–10 ppb of Ba was added to the actual samples. These findings indicate that polyatomic interferences of BaO or BaOH+ for Eu did not occur in this study.
Analyses of nutrients and DOC
To analyse nutrients (DIN, DIP and DSi), approximately 100 mL water samples was filtered through pre-combusted GF/F filters (Whatman, Kent, UK) and stored frozen in acid-washed 125 mL HDPE bottles (Nalgene, Rochester, NY, USA). In the laboratory, nutrients were measured using a Futura Plus auto-analyser (Alliance Instruments, Frepillon, France), which uses the Cu–Cd reduction column method for DIN and the molybdenum blue colorimetric method for DIP (Gordon et al. 1993). In addition, the certified reference material from MOOS-1 (National Research Council, Canada) was measured in each batch of the samples.
For DOC analyses, approximately 30 mL water samples was filtered through pre-combusted Whatman GF/F filters and acidified with 6 M HCl. The DOC samples were then heated in pre-combusted amber glass bottles (550°C for 5 h). A VCPH TOC analyser (Shimadzu, Kyoto, Japan) was used for the high-temperature combustion oxidation (HTCO) analysis of DOC (Dittmar et al. 2006). The detection limit was <5 µM. The accuracy was verified daily using a certified DOC seawater sample (deep seawater reference; 46–47 µM) obtained from the University of Miami. Our results were within 5% of the reference value.
Results and discussion
Concentrations of trace elements, nutrients and DOC in ice and snow
The concentrations of trace elements (Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, Pb, and REEs) in ice or snow were one to two orders of magnitude higher than those in seawater, whereas the concentrations of DIN, DIP, DSi and DOC in ice or snow were similar to, or much lower than, those in seawater (Tables 2, 3). Difference in Fe concentrations between ice and seawater was the largest: the concentrations of the total dissolved Fe in Marian Cove seawater were in the range of 0.1–3.8 nM (average 1.2±1.4 nM, n=8), while the concentrations of Fe in ice and snow samples were in the range of 2.1–38.1 nM (average 14±14 nM, n=5) and 2.6–29.2 nM (average 11±11 nM, n=5), respectively. These concentrations were within the range of those reported by previous studies for glacier samples, but our values were slightly lower than the average values (1–600 nM, average 33±21 nM, n=29) (Table 4), which could be related to the different methodologies used, the type of ice sampled (old glacier or recently formed ice) or the spatio-temporal scales investigated. Although there were no data for trace elements, the vertical distributions of the nutrients and the DOC in the upper layer (<30 m depth) were constant in this region.
Table 2 The concentrations of dissolved organic carbon (DOC), dissolved inorganic nitrogen (DIN), phosphorus (DIP), silicate (DSi) and trace elements in seawater, glacier floats and snow samples. | |||||||||||||||
Station no. | Salinity | Fe (nM) | DOC (µM) | DIN (µM) | DIP (µM) | DSi (µM) | Al (nM) | Cr (pM) | Mn (nM) | Co (pM) | Ni (nM) | Cu (nM) | Zn (nM) | Cd (pM) | Pb (pM) |
Seawater | |||||||||||||||
MC1 | 35.0 | 0.09 | 40 | 22 | 1.1 | 64 | 2.1 | 60 | 0.06 | 15 | 0.51 | 0.64 | 0.71 | 28 | 79 |
MC2 | 34.6 | 0.15 | 44 | 26 | 1.3 | 65 | 2.7 | 62 | 0.13 | 22 | 0.49 | 1.5 | 1.0 | 28 | 50 |
MC3 | 34.3 | 0.10 | 45 | 24 | 0.9 | 100 | 18 | 61 | 0.15 | 23 | 0.55 | 1.1 | 1.2 | 27 | 120 |
MC4 | 34.3 | 0.20 | 38 | 22 | 0.6 | 136 | 24 | 60 | 0.07 | 29 | 0.54 | 0.02 | 2.9 | 35 | 30 |
MC5 | 33.3 | 1.11 | 52 | 27 | 4.8 | 106 | 6.4 | 66 | 0.54 | 61 | 0.62 | 0.02 | 2.7 | 27 | 48 |
MC6 | 31.1 | 2.99 | 44 | 23 | 0.7 | 59 | 33 | 97 | 0.57 | 77 | 0.60 | 0.13 | 2.0 | 25 | 120 |
MC7 | 32.0 | 3.76 | 45 | 23 | 2.1 | 61 | 8.5 | 84 | 0.74 | 110 | 0.69 | 0.65 | 1.0 | 30 | 97 |
MC8 | 32.6 | 1.36 | 43 | 13 | 0.4 | 84 | 27 | 63 | 1.6 | 73 | 0.52 | 0.81 | 0.94 | 20 | 66 |
Floating iceberg | |||||||||||||||
1 | 0 | 13.7 | 20 | 5.5 | 0.5 | 1.3 | 11 | 116 | 223 | 1030 | 3.92 | 2.2 | 28 | 26 | 920 |
2 | 0 | 38.1 | 31 | 3.5 | 0.4 | 1.4 | 189 | 241 | 0.45 | 73 | 3.08 | 8.1 | 7.7 | 11 | 270 |
3 | 0 | 8.6 | 67 | 3.1 | 0.4 | 1.4 | 34 | 182 | 1.7 | 35 | 0.93 | 2.0 | 8.4 | 5.7 | 410 |
4 | 0 | 8.1 | 43 | 3.3 | 0.4 | 1.2 | 6.2 | 121 | 1.9 | 48 | 0.73 | 2.2 | 8.3 | 4 | 190 |
5 | 0 | 2.1 | 57 | 2.7 | 0.4 | 1.1 | 3.6 | 29 | 0.49 | 17 | 0.36 | 0.72 | 2.7 | 2.0 | 90 |
Snow melts | |||||||||||||||
1 | 0 | 12.5 | 26 | 6.6 | 0.2 | 30 | 115 | 59 | 11 | 260 | 1.33 | 0.87 | 1.5 | 13 | 160 |
2 | 0 | 4.0 | 31 | 7.2 | 0.1 | 20 | 102 | 139 | 8.0 | 2450 | 1.56 | 5.7 | 20 | 12 | 260 |
3 | 0 | 2.6 | 44 | 6.4 | 0.2 | 19 | 331 | 173 | 22 | 6830 | 4.25 | 14 | 20 | 25 | 340 |
4 | 0 | 6.1 | 28 | 6.2 | 0.2 | 18 | 12 | 129 | 3.7 | 170 | 0.18 | 0.82 | 2.4 | 4.3 | 88 |
5 | 0 | 29.2 | 43 | 5.7 | 0.2 | 32 | 219 | 258 | 17 | 5280 | 3.16 | 12 | 19 | 26 | 550 |
Table 3 The concentrations of dissolved rare earth elements (REE) in seawater, glacier floats and snow samples. | ||||||||||||||
| REE (pM) | |||||||||||||
Station no. | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Yb | Lu |
Seawater | ||||||||||||||
MC1 | 16.3 | 4.9 | 5.1 | 0.9 | 4.4 | 0.9 | 0.2 | 1.0 | 0.2 | 0.9 | 0.20 | 0.65 | 0.61 | 0.11 |
MC2 | 19.9 | 8.0 | 10.2 | 1.4 | 6.5 | 1.4 | 0.3 | 1.5 | 0.2 | 1.3 | 0.27 | 0.83 | 0.72 | 0.11 |
MC3 | 13.1 | 4.9 | 4.6 | 0.7 | 3.6 | 0.7 | 0.2 | 0.8 | 0.1 | 0.7 | 0.17 | 0.55 | 0.50 | 0.08 |
MC4 | 16.6 | 6.4 | 7.9 | 1.2 | 5.6 | 1.2 | 0.3 | 1.4 | 0.2 | 1.2 | 0.24 | 0.76 | 0.66 | 0.10 |
MC5 | 23.0 | 12.1 | 19.3 | 2.7 | 12.6 | 2.4 | 0.6 | 2.6 | 0.3 | 1.7 | 0.33 | 0.99 | 0.77 | 0.12 |
MC6 | 21.3 | 17.6 | 31.2 | 3.5 | 16.2 | 3.3 | 0.8 | 3.5 | 0.4 | 2.2 | 0.41 | 1.20 | 0.91 | 0.13 |
MC7 | 39.6 | 18.4 | 36.3 | 5.0 | 22.2 | 4.6 | 1.0 | 4.7 | 0.6 | 3.0 | 0.57 | 1.64 | 1.23 | 0.18 |
MC8 | 22.6 | 10.7 | 21.1 | 2.9 | 13.1 | 2.7 | 0.6 | 2.8 | 0.3 | 1.8 | 0.34 | 0.95 | 0.70 | 0.10 |
Floating iceberg | ||||||||||||||
1 | 827 | 215 | 435 | 41 | 136 | 31 | 9.0 | 35 | 9.6 | 34 | 13 | 34 | 24 | 6.1 |
2 | 14.6 | 9.9 | 19.7 | 2.7 | 12.5 | 3.0 | 0.9 | 2.8 | 0.4 | 2.0 | 0.33 | 0.92 | 0.66 | 0.10 |
3 | 4.3 | 3.2 | 5.1 | 0.7 | 4.0 | 0.8 | 0.2 | 0.8 | 0.1 | 0.5 | 0.10 | 0.28 | 0.21 | 0.02 |
4 | 4.9 | 3.6 | 5.1 | 0.7 | 3.3 | 0.7 | 0.2 | 0.8 | 0.1 | 0.5 | 0.10 | 0.29 | 0.20 | 0.03 |
5 | 2.1 | 1.5 | 1.8 | 0.2 | 1.2 | 0.3 | 0.1 | 0.3 | 0.0 | 0.2 | 0.04 | 0.12 | 0.08 | 0.01 |
Snow melts | ||||||||||||||
1 | 66 | 102 | 36 | 38 | 176 | 47 | 15 | 53 | 8.9 | 50 | 8.8 | 23 | 15 | 2.0 |
2 | 335 | 58 | 141 | 23 | 118 | 35 | 11 | 44 | 6.9 | 39 | 6.9 | 18 | 12 | 1.6 |
3 | 1090 | 187 | 482 | 76 | 382 | 114 | 36 | 139 | 23 | 130 | 23 | 59 | 41 | 5.1 |
4 | 21 | 4.1 | 9.1 | 1.5 | 8.1 | 2.3 | 0.7 | 3.0 | 0.4 | 2.6 | 0.5 | 1.2 | 0.8 | 0.1 |
5 | 719 | 128 | 313 | 50 | 254 | 74 | 23 | 93 | 15 | 84 | 15 | 39 | 27 | 3.4 |
Table 4 Dissolved Fe concentrations in glacier samples. | |||
Region (sampling year) | Location | Sample type | Fe (nM) |
Weddell Sea (2008–2009)a | 61–63°S, 48–52°W | Floating ice | 3.8–28.8 (13±12)b |
Gerlache Strait (Apr 1989)c | 64.9°S, 63.1°W | Floating ice | 25.9 |
Polar frontal region of Weddell Sea gyre (1992)d | 47°S to 59°S, 6°W | Floating ice | 20.4 |
|
| Ice core | 10.8–26.3 (19±11) |
Seymour Island, Weddell Seae | 64.3°S, 56.8°W | Floating Iceberg | 0.2–0.7 (0.4±0.3) |
Admiralty Strait off King George Islande | 62.2°S, 58.8° | Iceberg | 0.2–2.2 (1.2±1.4) |
Taylor Glacier and Canada Glaciere | 77.6–77.7°S, 162–163°E | Glacier | 0.1–3.8 (1.5±1.8) |
Southern branch of Antarctic circumpolar current region (1992)f | 52–58°S, 6°W | Floating ice | 30 |
Law Dome, Wilkes Land (1983)g | 66.1°S, 110.8°E | Ice core (BHC1) | 120 |
Law Dome, Wilkes Land (1988)g | 66.7°S, 113.2°E | Ice core (DE08) | 0.5–7.9 (2.9±2.7) |
Law Dome, Wilkes Land (1993)g | 66.7°S, 112.8°E | Ice core (DSS) | 0.6–2.5 (1.2±0.7) |
Marian Cove, King George Island, Barton Peninsula (2012)h | 62.2°S, 58.8°E | Floating ice | 2.1–38.1 (14±14) |
aLin et al. (2011). bOne abnormally high value (Fe: 607 nM) is excluded when calculating average concentration. cMartin (1990). dLöscher et al. (1997). eRaiswell et al. (2008). fde Baar et al. (1995). gEdwards et al. (1998). hThis study.
Concentrations of trace elements in seawater: inputs from glacier melting
The concentrations of trace elements (Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, Pb and REEs) in bay seawater are also listed in Tables 2 and 3. The concentrations of most trace elements (Al, Mn, Fe, Co, Cu, Zn, Cd, Pb and REEs) were found to be within the range of those reported from the major oceans (Westerlund & Öhman 1991; Sanudo-Wilhelmy et al. 2002; Saito et al. 2010), whereas those of Cr and Ni were an order of magnitude lower than those in the oligotrophic Southern Ocean (Fitzwater et al. 2000; Hendry et al. 2008). In general, Cd showed a good correlation with phosphate in the oceans (de Baar et al. 1994), with a ratio (mol:mol) of approximately 0.18×10−3–0.35×10−3 (de Baar et al. 1994). Because the average concentrations of in the seawater of this study area were 1.0±0.5 µM (mostly <1 µM), the relatively lower concentrations of Cd (10–100 nM) in this study could be related to biological removal occurring in the surface layer (as shown by Hendry et al. 2008) in the Antarctic region. As such, Löscher (1999) reported that the concentrations of Ni in seawater have positive relationship with major nutrients (especially phosphate). Therefore, we think that the relatively lower concentrations of Cr, Ni, and Cd, which are generally known as nutrient-like elements, in our study region could be associated with larger biological removal during the austral summer.
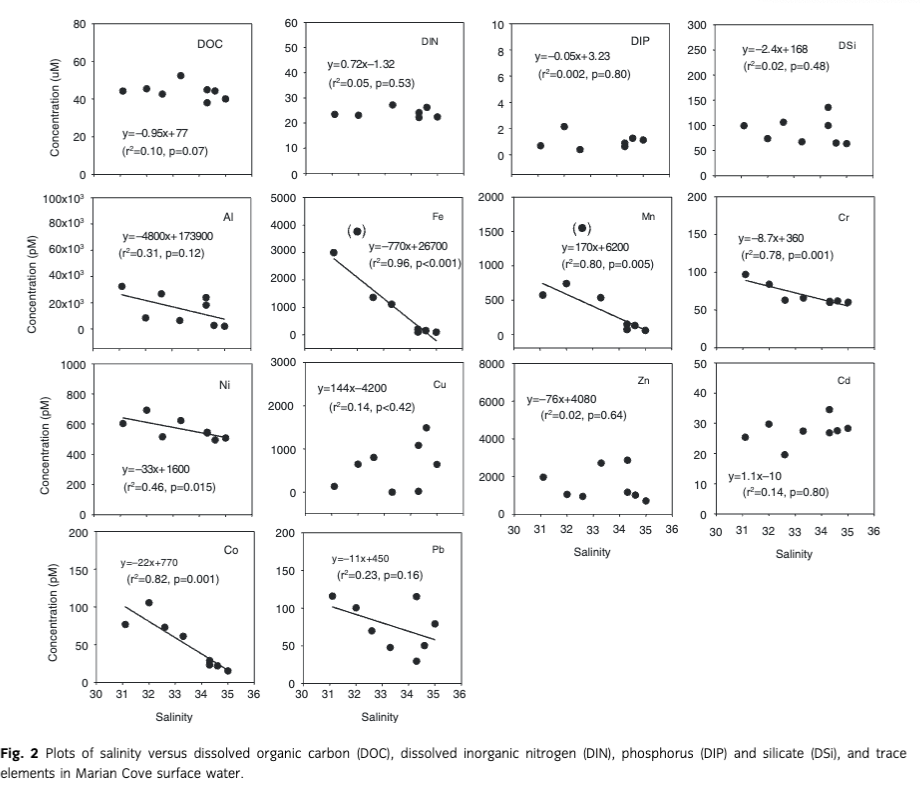
The concentrations of trace elements (Al, Cr, Mn, Fe, Co, Ni, Pb and REEs) in seawater showed significant negative relationships with salinities (Figs. 2, 3). The salinities of seawater decreased from 35.0 to 31.0 at locations near a huge ice valley and at coastal beaches where a large volume of GMW discharges into the sea. When extrapolating the linear plot to a zero salinity by assuming the conservative mixing of dissolved Fe in GMW and particles in coastal water, the dissolved Fe concentration was determined to be about 27±3 nM, which represents the dissolved Fe endmember of all fresh GMW entering into the bay (Fig. 2). Khim et al. (1997) estimated that the freshwater in this region is almost exclusively derived from GMW (d18O=−44) during the summer, based on the relationship between d18O and salinity. All the isotopic data for Marian Cove were distributed along the line of mixing between isotopically lighter freshwater and isotopically heavier saline seawater. This endmember is therefore likely to be a representative value of dissolved Fe in the GMW in this region.
Endmembers of GMW-derived Fe and other trace elements in seawater
This endmember concentration of dissolved Fe falls into the range of that in the ice (2.1–38.1 nM) and snow (2.6–29.2 nM) samples collected in the same region but slightly higher than the average values (14±14 nM, n=5 for ice; 11±11 nM, n=5 for snow). Liu & Millero (2002) reported that the solubility of Fe in seawater could be increased by up to 60% (0.25–0.4 nM) than that in freshwater due to higher ionic strengths. In addition, Thuróczy et al. (2012) suggested that the presence of organic ligands could also increase the solubility of Fe in seawater. The Fe endmember that is higher than the Fe concentration in ice is therefore considered to be related to the additional Fe released from particles in ice and shallow sediments.
The Fe concentrations in our ice samples are within the range of those reported by previous studies for glacier samples, but slightly lower than the average values (1–600 nM, mean=33±21 nM, n=29; Table 4). Although Lin et al. (2011) did not quantify the Fe inputs from glacier melting water and particles, they observed the relationship between Fe and salinity in the Weddell Sea, indicating the Fe concentration of GMW to be 45±13 nM. These discrepancies are considered to be related to the large differences in the methodologies, the type of ice samples (old glacier or recently formed ice), and the spatio-temporal scales sampling. Our endmember value could be slightly underestimated owing to various Fe sinks such as phytoplankton uptake, settling, and scavenging, although Fe shows a strong negative correlation with salinity.
We also estimated the endmember concentrations of other trace elements in ice samples using the same method. The extrapolated endmember concentrations of Al, Mn, Cr, Ni, Co and Pb were 174±95 nM, 6.1±1.3 nM, 0.36±0.06 nM, 1.7±0.5 nM, 0.77±0.14 and 0.45±0.28 nM, respectively. Although trace elements data in glacial ice are scarce, we compare our endmember concentrations to those in ice core samples. Our endmembers of Cr (360±60 pM) and Pb (450±280 pM) were similar to, or significantly higher than, those of previously reported values (180–710 pM for Cr and 0.070–12 nM for Pb from Dome C and the Low Dome ice core, respectively; Boutron et al. 1994; Gabrielli et al. 2005). The endmember of Al in our study was estimated to be 174 nM, which is one to two orders of magnitude higher than that in the Low Dome ice core (Hong et al. 1998). This difference is likely due to the different types of ice samples and spatio-temporal variations.
Influence of GMW-driven trace element fluxes on their inventories in seawater
If we assume that the average salinity in Marian Cove is 33, the amount of GMW in the surface waters (<30 m depth) of Marian Cove is estimated to be approximately 7.2×106 m3. Assuming that the average salinity in Marian Cove is 33, we calculated the contribution of GMW in the inventory of trace elements in Marian Cove, based on the correlations with salinity. The calculated average concentrations of these elements in cove water were thus increased by approximately eight-fold for Al, 1.2-fold for Cr, 10-fold for Mn, 18-fold for Fe, four-fold for Co, 1.2-fold for Ni, 2.9-fold for Pb, and 1.9-fold for REEs, relative to the background seawater (with no GMW input) (Fig. 4). In contrast, we did not find any appreciable inputs of Cu, Cd, nutrients (DIN, DIP and DSi), and DOC from GMW to the bay water (ratios ≤1, Fig. 4) as predicted from the lower concentrations of these chemical components in ice/snow samples than in seawater.
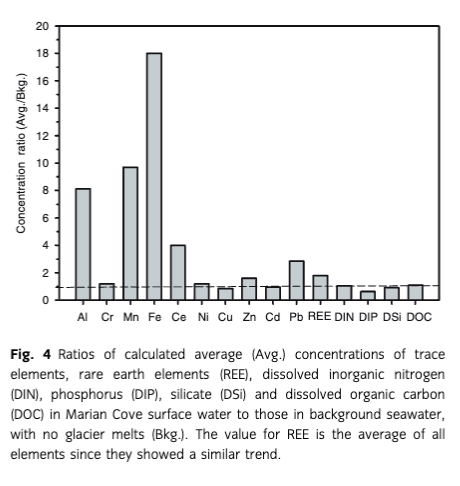
The calculated average Fe concentration enhanced by GMW input was about 1.6 nM (Fig. 4), which was much higher than that in the background seawater concentration (0.09 nM Fe with no GMW input). Considering that the fraction of fresh glacier melts (7.2×106 m3) accounts for only about 5.7% of the total Marian Cove surface water (1.2×108 m3), the impact of GMW to the Fe inventory is striking. Our calculations covered the trace element budgets of the entire cove area resulting from glacial melts and dissolution from (glacial) particles. These results suggest that Antarctic glacier melting has a significant influence on the budgets of trace elements (particularly Fe).
REEs as tracers of GMW-derived trace elements
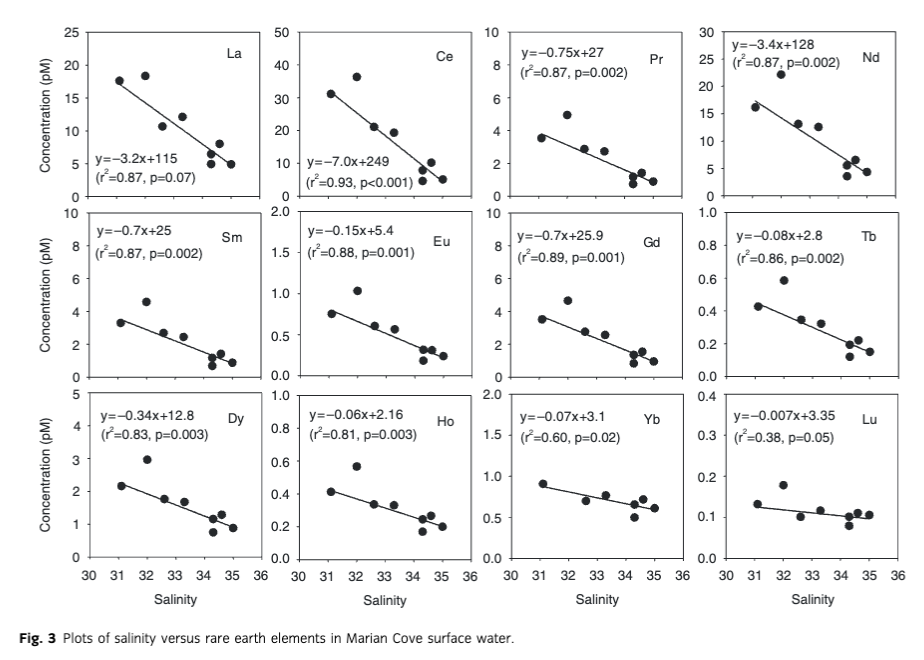
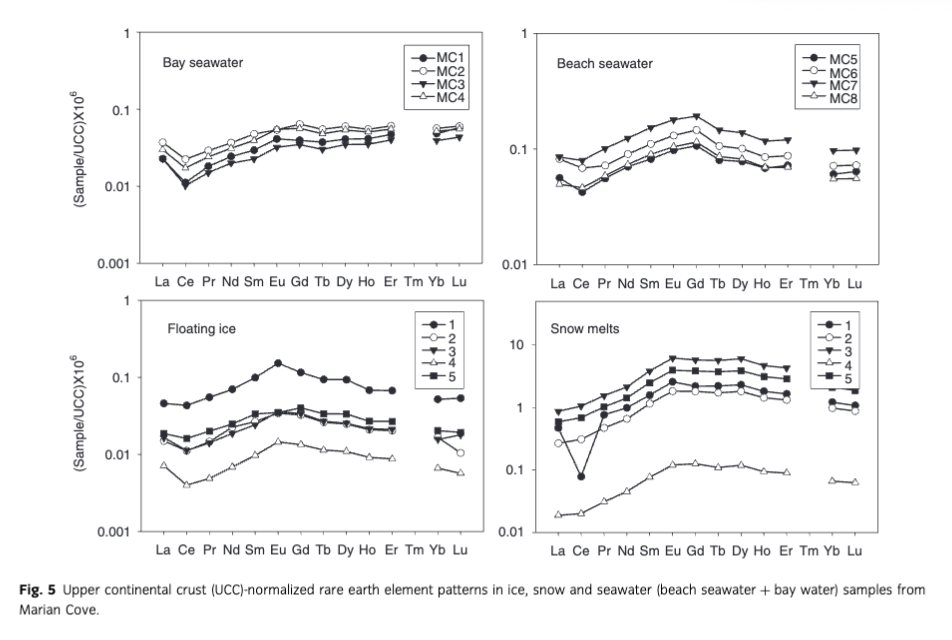
As evidence of terrestrial source inputs for REEs, we found a substantial elevation of MREE in the bay seawater when normalized for the upper continental crust (Taylor & McLennan 1985). This MREE enrichment, relative to the typical ocean pattern (Elderfield et al. 1990; Nozaki et al. 2000), is evidently related to glacier melting as there was a strong relationship between salinity and REEs (Fig. 3). The MREE enrichment pattern was even clearer in beach seawater samples (Fig. 5). All our ice floats and snow exhibited MREE enrichment, which could be from Patagonian origin because this pattern resembles those from volcanic rocks sampled in that region (Gaiero et al. 2004) and/or those shown by South American dust (Gabrielli et al. 2010).
Implication: GMW as a source of Fe in the Southern Ocean
Although a large fraction of Fe supplied into the coastal ocean probably settles to bottom sediments before being utilized by phytoplankton, Fe adsorbed onto sediments can be returned to the water column around the Antarctic Peninsula through sedimentary diagenesis or re-suspension (de Jong et al. 2013). This remobilized Fe can also be transported to offshore regions through lateral water mixing. Previous studies (Dulaiova et al. 2009; Ardelan et al. 2010) showed that the transport of the Antarctic shelf-water to the Southern Ocean is an important mechanism for Fe delivery to the offshore region. For example, Dulaiova et al. (2009) reported that the Fe flux through lateral transport to the Drake Passage is 4.0×107 mol Fe yr−1, which is much larger than the vertical flux of dust-borne Fe. Ardelan et al. (2010) also suggested that this supply of Fe by lateral transport contributes significantly to high phytoplankton biomass in the Scotia Sea region. More recently, de Jong et al. (2012) showed that almost a half (54%) of the total Fe flux to the Atlantic sector of the Southern Ocean is transported through horizontal advection.
Recent studies have also shown that global warming may have caused an increase in the disintegration of the ice shelves along the Antarctic Peninsula by up to 13% during the last two decades (Rignot et al. 2011). Our results imply that the increase of Fe in association with glacier melting may enhance the future biological productivity of the Southern Ocean.
Conclusions
This study found that the inventories of some trace elements (Al, Fe, Mn, Cr, Ni, Co, Pb, and REEs) in seawater were increased up to 18-fold in Marian Cove, Antarctica (62°S), by GMW inputs. The GMW inputs resulted in substantially higher MREE in seawater, a pattern indicative of terrestrial source inputs. Our results imply that the modern rates of glacier/ice melting in Antarctica may contribute significantly to the budgets of trace elements in coastal seawater. Further extensive studies are necessary in order to quantify the fluxes of trace elements, particularly Fe, caused by GMW transport to the polar oceans and to evaluate the biogeochemical consequences of such fluxes to the ocean require further investigation.
Acknowledgements
We are grateful to three anonymous reviewers for providing insightful comments and suggestions that improved this manuscript. We also thank all workers at the Korea Polar Research Institute and the Korean Antarctic research base, King Sejong, who helped with field work and sampling. This work was supported by a National Research Foundation of Korea grant funded by the Korean government (NRF-2013R1A2A1A05004343) and a grant from the Korea Polar Research Institute for the project titled Impacts of Ocean acidification on Calcifying Organisms in the Antarctic Marine Ecosystem (PE14150). IK was supported by scholarships from BK21.
References
- Ahn I.Y., Chung H., Kang J.S. & Kang S.H. 1997. Diatom composition and biomass variability in nearshore waters of Maxwell Bay, Antarctica, during the 1992/1993 austral summer. Polar Biology 17, 123–130. Publisher Full Text
- Alderkamp A.C., Mills M.M., van Dijken G.L., Laan P., Thuróczy C.E., Gerringa L.J.A., de Baar H.J.W., Payne C.D., Visser R.J.W. & Buma A.G.J. 2012. Iron from melting glaciers fuels phytoplankton blooms in the Amundsen Sea (Southern Ocean): phytoplankton characteristics and productivity. Deep-Sea Research Part II 71, 32–48. Publisher Full Text
- Ardelan M.V., Holm-Hansen O., Hewes C.D., Reiss C.S., Silva N.S., Dulaiova H., Steinnes E. & Sakshaug E. 2010. Natural iron enrichment around the Antarctic Peninsula in the Southern Ocean. Biogeosciences 7, 11–25. Publisher Full Text
- Bhatia M.P., Kujawinski E.B., Das S.B., Breier C.F., Henderson P.B. & Charette M.A. 2013. Greenland meltwater as a significant and potentially bioavailable source of iron to the ocean. Nature Geoscience 6, 274–278. Publisher Full Text
- Bindschadler R. & Rignot E. 2001. “Crack!” in the polar night. Eos, Transactions of the American Geophysical Union 82, 497–505. Publisher Full Text
- Boutron C.F., Candelone J.P. & Hong S. 1994. Past and recent changes in the large-scale tropospheric cycles of lead and other heavy metals as documented in Antarctic and Greenland snow and ice: a review. Geochimica et Cosmochimica Acta 58, 3217–3225. Publisher Full Text
- Buma A.G.J., de Baar H.J.W., Nolting R.F. & van Bennekom A.J. 1991. Metal enrichment experiments in the Weddell–Scotia seas: effects of iron and manganese on various plankton communities. Limnology and Oceanography 36, 1865–1878. Publisher Full Text
- Coale K.H., Wang X., Tanner S.J. & Johnson K.S. 2003. Phytoplankton growth and biological response to iron and zinc addition in the Ross Sea and Antarctic Circumpolar Current along 170 W. Deep-Sea Research Part II 50, 635–653. Publisher Full Text
- Cook A.J. & Vaughan D.G. 2009. Overview of areal changes of the ice shelves on the Antarctic Peninsula over the past 50 years. The Cryosphere Discussions 3, 579–630. Publisher Full Text
- de Baar H.J.W., de Jong J.T.M., Bakker D.C.E., Löscher B.M., Veth C., Bathmann U. & Smetacek V. 1995. Importance of iron for plankton blooms and carbon dioxide drawdown in the Southern Ocean. Nature 373, 412–415. Publisher Full Text
- de Baar H.J.W., Saager P.M., Nolting R.F. & van der Meer J. 1994. Cadmium versus phosphate in the world ocean. Marine Chemistry 46, 261–281. Publisher Full Text
- de Jong J., Schoemann V., Lannuzel D., Croot P., de Baar H. & Tison J.L. 2012. Natural iron fertilization of the Atlantic sector of the Southern Ocean by continental shelf sources of the Antarctic Peninsula. Journal of Geophysical Research—Biogeosciences 117, G01029, doi: 10.1029/2011JG001679.
- de Jong J., Schoemann V., Maricq N., Mattielli N., Langhorne P., Haskell T. & Tison J.L. 2013. Iron in land-fast sea ice of McMurdo Sound derived from sediment resuspension and wind-blown dust attributes to primary productivity in the Ross Sea, Antarctica. Marine Chemistry 157, 24–40. Publisher Full Text
- Dittmar T., Hertkorn N., Kattner G. & Lara R.J. 2006. Mangroves, a major source of dissolved organic carbon to the oceans. Global Biogeochemical Cycles 20, GB1012, doi: 10.1029/2005GB002570. Publisher Full Text
- Dulaiova H., Ardelan M., Henderson P.B. & Charette M.A. 2009. Shelf-derived iron inputs drive biological productivity in the southern Drake Passage. Global Biogeochemical Cycles 23, GB4014, doi: 10.1029/2008GB003406. Publisher Full Text
- Edwards R., Sedwick P.N., Morgan V., Boutron C.F. & Hong S. 1998. Iron in ice cores from Law Dome, East Antarctica: implications for past deposition of aerosol iron. Annals of Glaciology 27, 365–370.
- Elderfield H., Upstill-Goddard R. & Sholkovitz E.R. 1990. The rare earth elements in rivers, estuaries, and coastal seas and their significance to the composition of ocean waters. Geochimica et Cosmochimica Acta 54, 971–991. Publisher Full Text
- Fitzwater S., Johnson K., Gordon R., Coale K. & Smith W. Jr. 2000. Trace metal concentrations in the Ross Sea and their relationship with nutrients and phytoplankton growth. Deep-Sea Research Part II 47, 3159–3179. Publisher Full Text
- Gabrielli P., Barbante C., Boutron C., Cozzi G., Gaspari V., Planchon F., Ferrari C., Turetta C., Hong S. & Cescon P. 2005. Variations in atmospheric trace elements in Dome C (East Antarctica) ice over the last two climatic cycles. Atmospheric Environment 39, 6420–6429. Publisher Full Text
- Gabrielli P., Wegner A., Petit J.R., Delmonte B., de Deckker P., Gaspari V., Fischer H., Ruth U., Kriews M. & Boutron C. 2010. A major glacial–interglacial change in aeolian dust composition inferred from rare earth elements in Antarctic ice. Quaternary Science Reviews 29, 265–273. Publisher Full Text
- Gaiero D.M., Depetris P.J., Probst J.L., Bidart S.M. & Leleyter L. 2004. The signature of river- and wind-borne materials exported from Patagonia to the southern latitudes: a view from REEs and implications for paleoclimatic interpretations. Earth and Planetary Science Letters 219, 357–376. Publisher Full Text
- Gerringa L.J.A., Alderkamp A.C., Laan P., Thuroczy C.E., de Baar H.J.W., Mills M.M., van Dijken G.L., van Haren H. & Arrigo K.R. 2012. Iron from melting glaciers fuels the phytoplankton blooms in Amundsen Sea (Southern Ocean): iron biogeochemistry. Deep-Sea Research Part II 71, 16–31. Publisher Full Text
- Gordon L.I., Jennings J.C. Jr., Ross A.A. & Krest J.M. 1993. A suggested protocol for continuous flow automated analysis of seawater nutrients (phosphate, nitrate, nitrite and silicic acid) in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study. Methods Manual WHPO91–1. Corvallis, OR: World Ocean Circulation Experiment Hydrographic Program Office, Oregon State University.
- Hendry K.R., Rickaby R.E., de Hoog J., Weston K. & Rehkämper M. 2008. Cadmium and phosphate in coastal Antarctic seawater: implications for Southern Ocean nutrient cycling. Marine Chemistry 112, 149–157. Publisher Full Text
- Hong S., Boutron C.F., Edwards R. & Morgan V.I. 1998. Heavy metals in Antarctic ice from Law Dome: initial results. Environmental Research 78, 94–103. PubMed Abstract | Publisher Full Text
- Jacobs S.S., Jenkins A., Giulivi C.F. & Dutrieux P. 2011. Stronger ocean circulation and increased melting under Pine Island Glacier ice shelf. Nature Geoscience 4, 519–523. Publisher Full Text
- Jeong J., Kim G. & Han S. 2012. Influence of trace element fluxes from submarine groundwater discharge (SGD) on their inventories in coastal waters off volcanic island, Jeju, Korea. Applied Geochemistry 27, 37–43. Publisher Full Text
- Khazendar A., Schodlok M.P., Fenty I., Ligtenberg S.R.M., Rignot E. & van den Broeke M.R. 2013. Observed thinning of Totten Glacier is linked to coastal polynya variability. Nature Communications 4, article no. 2857, doi: 10.1038/ncomms3857. Publisher Full Text
- Khim B.K., Park B.K. & Yoon H.I. 1997. Oxygen isotopic compositions of seawater in the Maxwell Bay of King George Island, West Antarctica. Geosciences Journal 1, 115–121. Publisher Full Text
- Kim I. & Kim G. 2011. Large fluxes of rare earth elements through submarine groundwater discharge (SGD) from a volcanic island, Jeju, Korea. Marine Chemistry 127, 12–19. Publisher Full Text
- Klöser H., Ferreyra G., Schloss I., Mercuri G., Laturnus F. & Curtosi A. 1993. Seasonal variation of algal growth conditions in sheltered Antarctic bays: the example of Potter Cove (King George Island, South Shetlands). Journal of Marine Systems 4, 289–301. Publisher Full Text
- Lee J.M., Boyle E.A., Echegoyen-Sanz Y., Fitzsimmons J.N., Zhang R. & Kayser R.A. 2011. Analysis of trace metals (Cu, Cd, Pb, and Fe) in seawater using single batch nitrilotriacetate resin extraction and isotope dilution inductively coupled plasma mass spectrometry. Analytica Chimica Acta 686, 93–101. PubMed Abstract | Publisher Full Text
- Lewis B.L. & Landing W.M. 1991. The biogeochemistry of manganese and iron in the Black Sea. Deep-Sea Research Part A 38, S773–S803. Publisher Full Text
- Lin H., Rauschenberg S., Hexel C.R., Shaw T.J. & Twining B.S. 2011. Free-drifting icebergs as sources of iron to the Weddell Sea. Deep-Sea Research Part II 58, 1392–1406. Publisher Full Text
- Liu X. & Millero F.J. 2002. The solubility of iron in seawater. Marine Chemistry 77, 43–54. Publisher Full Text
- Long D.G., Ballantyn J. & Bertoia C. 2002. Is the number of Antarctic icebergs really increasing? Eos, Transactions of the American Geophysical Union 83, 469–474. Publisher Full Text
- Löscher B.M. 1999. Relationships among Ni, Cu, Zn, and major nutrients in the Southern Ocean. Marine Chemistry 67, 67–102. Publisher Full Text
- Löscher B.M., de Baar H.J.W., de Jong J.T.M., Veth C. & Dehairs F. 1997. The distribution of Fe in the Antarctic circumpolar current. Deep-Sea Research Part II 44, 143–187. Publisher Full Text
- Luckman A., Padman L. & Jansen D. 2010. Persistent iceberg groundings in the western Weddell Sea, Antarctica. Remote Sensing of Environment 114, 385–391. Publisher Full Text
- Maher B.A. & Dennis P.F. 2001. Evidence against dust-mediated control of glacial–interglacial changes in atmospheric CO2. Nature 411, 176–180. PubMed Abstract | Publisher Full Text
- Martin J.H. 1990. Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography 5, 1–13. Publisher Full Text
- Milne A., Landing W., Bizimis M. & Morton P. 2010. Determination of Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb in seawater using high resolution magnetic sector inductively coupled mass spectrometry (HR-ICP-MS). Analytica Chimica Acta 665, 200–207. PubMed Abstract | Publisher Full Text
- Nozaki Y., Lerche D., Alibo D.S. & Tsutsumi M. 2000. Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and In. Geochimica et Cosmochimica Acta 64, 3975–3982. Publisher Full Text
- Öztürk M. 1995. Trends of trace metal (Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb) distributions at the oxic–anoxic interface and in sulfidic water of the Drammensfjord. Marine Chemistry 48, 329–342. Publisher Full Text
- Öztürk M., Bizsel N. & Steinnes E. 2003. Iron speciation in eutrophic and oligotrophic Mediterranean coastal waters; impact of phytoplankton and protozoan blooms on iron distribution. Marine Chemistry 81, 19–36. Publisher Full Text
- Pritchard H.D., Ligtenberg S.R.M., Fricker H.A., Vaughan D.G., van den Broeke M.R. & Padman L. 2012. Antarctic ice-sheet loss driven by basal melting of ice shelves. Nature 484, 502–505. PubMed Abstract | Publisher Full Text
- Raiswell R. 2011. Iceberg-hosted nanoparticulate Fe in the Southern Ocean: mineralogy, origin, dissolution kinetics and source of bioavailable Fe. Deep-Sea Research Part II: Topical Studies in Oceanography 58, 1364–1375. Publisher Full Text
- Raiswell R., Benning L.G., Tranter M. & Tulaczyk S. 2008. Bioavailable iron in the Southern Ocean: the significance of the iceberg conveyor belt. Geochemical Transactions 9, 7, doi: 10.1186/1467-4866-9-7. Publisher Full Text
- Rignot E., Jacobs S., Mouginot J. & Scheuchl B. 2013. Ice-shelf melting around Antarctica. Science 341, 266–270. PubMed Abstract | Publisher Full Text
- Rignot E. & Steffen K. 2008. Channelized bottom melting and stability of floating ice shelves. Geophysical Research Letters 35, article no. 7, doi: 10.1029/2007GL031765.
- Rignot E. & Thomas R.H. 2002. Mass balance of polar ice sheets. Science 297, 1502–1506. PubMed Abstract | Publisher Full Text
- Rignot E., Velicogna I., van den Broeke M.R., Monaghan A. & Lenaerts J. 2011. Acceleration of the contribution of the Greenland and Antarctic ice sheets to sea level rise. Geophysical Research Letters 38, L05503, doi: 10.1029/2011GL046583. Publisher Full Text
- Saito M.A., Goepfert T.J., Noble A.E., Bertrand E.M., Sedwick P.N. & DiTullio G.R. 2010. A seasonal study of dissolved cobalt in the Ross Sea, Antarctica: micronutrient behavior, absence of scavenging, and relationships with Zn, Cd, and P. Biogeosciences 7, 4059–4082. Publisher Full Text
- Sanudo-Wilhelmy S., Olsen K., Scelfo J., Foster T. & Flegal A. 2002. Trace metal distributions off the Antarctic Peninsula in the Weddell Sea. Marine Chemistry 77, 157–170. Publisher Full Text
- Scambos T.A., Hulbe C., Fahnestock M. & Bohlander J. 2000. The link between climate warming and break-up of ice shelves in the Antarctic Peninsula. Journal of Glaciology 46, 516–530. Publisher Full Text
- Schodlok M.P., Hellmer H.H., Rohardt G. & Fahrbach E. 2006. Weddell Sea iceberg drift: five years of observations. Journal of Geophysical Research—Oceans 111, article no. C06018, doi: 10.1029/2004JC002661.
- Schwarz J.N. & Schodlok M.P. 2009. Impact of drifting icebergs on surface phytoplankton biomass in the Southern Ocean: ocean colour remote sensing and in situ iceberg tracking. Deep-Sea Research Part I 56, 1727–1741. Publisher Full Text
- Shaw T.J., Raiswell R., Hexel C.R., Vu H.P., Moore W.S., Dudgeon R. & Smith K.R. Jr. 2011. Input, composition, and potential impact of terrigenous material from free-drifting icebergs in the Weddell Sea. Deep-Sea Research Part II 58, 1376–1383. Publisher Full Text
- Shim J.G., Kang Y.C., Kang D.J. & Han M.W. 2011. Fluxes and budgets of biogenic elements at the sediment–water interface of Marian Cove, King George Island. Antarctic Science 1, 1–11.
- Smith K.L. Jr., Robison B.H., Helly J.J., Kaufmann R.S., Ruhl H.A., Shaw T.J., Twining B.S. & Vernet M. 2007. Free-drifting icebergs: hot spots of chemical and biological enrichment in the Weddell Sea. Science 317, 478–482. PubMed Abstract | Publisher Full Text
- Statham P.J., Skidmore M. & Tranter M. 2008. Inputs of glacially derived dissolved and colloidal iron to the coastal ocean and implications for primary productivity. Global Biogeochemical Cycles 22, GB3013, doi: 10.1029/2007GB003106. Publisher Full Text
- Sunda W.G. & Huntsman S.A. 1997. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 390, 389–392. Publisher Full Text
- Taylor S.R. & McLennan S.M. 1985. The continental crust: its composition and evolution. Oxford: Blackwell Scientific.
- Thuróczy C.E., Alderkamp A.C., Laan P., Gerringa L.J., Mills M.M., Van Dijken G.L., de Baar H.J.W. & Arrigo K.R. 2012. Key role of organic complexation of iron in sustaining phytoplankton blooms in the Pine Island and Amundsen polynyas (Southern Ocean). Deep-Sea Research Part II 71, 49–60.
- Velicogna I. & Wahr J. 2006. Measurements of time-variable gravity show mass loss in Antarctica. Science 311, 1754–1756. PubMed Abstract | Publisher Full Text
- Westerlund S. & Öhman P. 1991. Cadmium, copper, cobalt, nickel, lead, and zinc in the water column of the Weddell Sea, Antarctica. Geochimica et Cosmochimica Acta 55, 2127–2146. Publisher Full Text
- Williams R.N., Rees W.G. & Young N.W. 1999. A technique for the identification and analysis of icebergs in synthetic aperture radar images of Antarctica. International Journal of Remote Sensing 20, 3183–3199. Publisher Full Text
- Yoo K.C., Yoon H.I., Oh J.K., Kang C.Y. & Khim B.K. 2000. Water column structure and dispersal pattern of suspended particulate matter (SPM) in a floating ice-dominated fjord. Marian Cove, Antarctica during austral summer. The Sea 5, 295–304.
- Yoon H.I., Park B.K., Domack E.W. & Kim Y. 1998. Distribution and dispersal pattern of suspended particulate matter in Maxwell Bay and its tributary, Marian Cove, in the South Shetland Islands, West Antarctica. Marine Geology 152, 261–275. Publisher Full Text