3D tooth microwear texture analysis in fishes as a test of dietary hypotheses of durophagy


Published: Jan. 1, 2015
Latest article update: Nov. 17, 2022
Abstract
An understanding of how extinct animals functioned underpins our understanding of past evolutionary events, including adaptive radiations, and the role of functional innovation and adaptation as drivers of both micro- and macroevolution. Yet analysis of function in extinct animals is fraught with difficulty. Hypotheses that interpret molariform teeth in fishes as evidence of durophagous (shell-crushing) diets provide a good example of the particular problems inherent in the methods of functional morphology. This is because the assumed close coupling of form and function upon which the approach is based is weakened by, among other things, behavioural flexibility and the absence of a clear one to one relationship between structures and functions. Here we show that ISO 25178-2 standard parameters for surface texture, derived from analysis of worn surfaces of molariform teeth of fishes, vary significantly between species that differ in the amount of hard-shelled prey they consume. Two populations of the Sheepshead Seabream (Archosargus probatocephalus) were studied. This fish is not a dietary specialist, and one of the populations is known to consume more vegetation and less hard-shelled prey than the other; this is reflected in significant differences in their microwear textures. The Archosargus populations differ significantly in their microwear from the specialist shell-crusher Anarhichas lupus (the Atlantic Wolffish). Multivariate analysis of these three groups of fishes lends further support to the relationship between diet and tooth microwear, and provides robust validation of the approach. Application of the multivariate models derived from microwear texture in Archosargus and Anarhichas to a third fish species—the cichlid Astatoreochromis alluaudi—successfully separates wild caught fish that ate hard-shelled prey from lab-raised fish that did not. This cross-taxon validation demonstrates that quantitative analysis of tooth microwear texture can differentiate between fishes with different diets even when they range widely in size, habitat, and in the structure of their trophic apparatus. The approach thus has great potential as an additional tool for dietary analysis in extant fishes, and for testing dietary hypotheses in ancient and extinct species.
Keywords
3D tooth, extinct animals, Hypotheses, fishes, test of dietary hypotheses of durophagy, evolutionary events
1. Introduction
In the context of the past, functional morphology is widely used as an approach to inferring how ancient animals functioned, shedding light on aspects of behaviour and interactions with the environment. An understanding of function also underpins our understanding of past evolutionary events, including adaptive radiations, and the role of functional innovation and adaptation as drivers of both micro- and macroevolutionary patterns and processes. This approach to function, of course, requires the relationship between form and function to be well understood, and this has been the goal of decades of functional and biomechanical analysis of extant animals. But structure and function can be less well coupled than is assumed by uncritical application of functional morphology to extinct organisms. Expression of morphological traits is influenced by a variety of factors, including some that are closely linked to environmental conditions (responses to selection, phenotypic plasticity and non-selective effects; e.g. Langerhans et al 2003, Binning and Chapman 2010, Binning et al 2010), and others that are not directly linked (genetic and developmental factors). In extant fishes, these morphological traits form the basis of models with which to estimate prey capture and prey processing efficiency, but prey availability (as a function of predation or seasonality) and biological interactions, like intraspecific and interspecific competition for food resources, also shape the diet.
Although some studies of fishes have highlighted interspecific differences in diet associated with diverging morphologies (Wainwright and Richard 1995, Bellwood 2003, Cochran-Biederman and Winemiller 2010), at the inter-population scale, diet and ecomorphology do not necessarily match (Cutwa and Turingan 2000, Binning and Chapman 2010). Such variability, along with the observed many-to-one mapping of form to function in fishes (i.e. the fact that several morphological combinations have similar functional properties Wainwright et al 2005), explains why a direct link between morphological features and feeding performance (Wainwright and Richard 1995) or observed diet (Binning et al 2009) has rarely been observed.
For all these reasons, analysis of feeding and diet in fishes provides a widely cited, classic illustration of the pitfalls and limitations of functional morphology applied to extinct organisms (Lauder 1995), and how, without the possibility of direct observation or experimental evidence of function, inferences of specific roles for particular fossil structures are likely to be weak. Here we explore the relationship between diet and tooth microwear in shell crushing fishes in order to test the hypothesis that 3D microwear texture analysis can provide a proxy for direct observation of diet and feeding in fossil fishes, as suggested by Purnell et al (2012). Shell crushing in fishes provided one of Lauder's cautionary tales (1995), and there is clear evidence of mismatch between the consumption of shell-bearing food items and apparent morphological specialisation for shell crushing (Cutwa and Turingan 2000, Binning and Chapman 2010). This phenomenon—that morphological specialists often behave like generalists—is generally referred to as Liem's paradox (Liem 1973, 1980), particularly in the context of dietary preferences of fishes.
The extant sheepshead seabream (Archosargus probatocephalus, Walbaum 1792) exemplifies many of the difficulties of inferring diet from functional morphological analysis. This species exhibits anatomical traits consistent with the hypothesis that it is a specialist shell-crusher (Hernandez and Motta 1997 and references therein), but in some ecosystems sheepshead are the main plant consumer (Castillo-Rivera et al 2007), and the species is known to exhibit significant between-population differences in diet in lagoons from the same region (Cutwa and Turingan 2000).
Although quantitative analysis of dental microwear is a technique widely used for dietary discrimination in fossil and extant mammals (e.g., Walker et al 1978, Scott et al 2005, Mainland 2006, Gill et al 2014), it has rarely been applied to fishes. Purnell et al (2006, 2007) conducted a 2D analysis of microwear, based on operator scoring of microwear features, of extant and fossil threespine sticklebacks, Gasterosteus aculeatus (Purnell et al 2006), revealing that despite biomechanical and developmental differences between actinopterygian and mammalian teeth (e.g. polyphyodonty, non-occlusal tooth contact) the method provided a reliable guide to discriminate between sticklebacks from different trophic niches. The only previous application of 3D texture analysis of tooth microwear in fishes is a proof of concept study of oral and pharyngeal teeth in cichlids (Purnell et al 2012). Nevertheless, fishes represent good models with which to test microwear approaches for a number of reasons. They do not employ digestive strategies similar to those of the ruminants (returning the bolus back into the mouth to mechanically process it several times) (see Mountfort et al 2002), and while some fishes spit out broken shells as they process their food, they do it before further ingestion, hence there is no contact between teeth and enzymes from the post-pharyngeal digestive tract. Food items are thus the main influence on dental surfaces in the fishes.
Our objective with this study is to explore the potential for quantitative 3D texture analysis of tooth microwear to discriminate between populations of wild-caught fishes with differences in diet by testing the following hypotheses:
Hypothesis 1: Within a species, dental microwear texture analysis can discriminate between two morphologically similar populations which have different diets. This is tested through analysis of two populations of Archosargus probatocephalus that differ in their degree of herbivory and durophagy.
Hypothesis 2: Analysis of dental microwear texture can discriminate between a specialised shell-crusher and more opportunistic generalists which consume some hard-shelled prey. This is tested by analysis of teeth from wild-caught Anarhichas lupus (the Atlantic wolffish), and from the two Archosargus populations.
We further test the general validity of the hypothesis that analysis of dental microwear texture provides reliable evidence of diet through a cross-taxon validation, assessing whether microwear texture correlates with diet across different environments (freshwater, shallow marine, deeper marine) body sizes, and tooth locations (oral and pharyngeal jaws). This is achieved by using multivariate analyses of microwear texture in Archosargus and Anarhichas to predict the diet of individuals of the cichlid Astatoreochromis alluaudi. These individuals have known dietary differences, so comparison of the known diet and that predicted by the multivariate model provides robust validation of the method.
2. Method and materials
2.1. Populations sampled
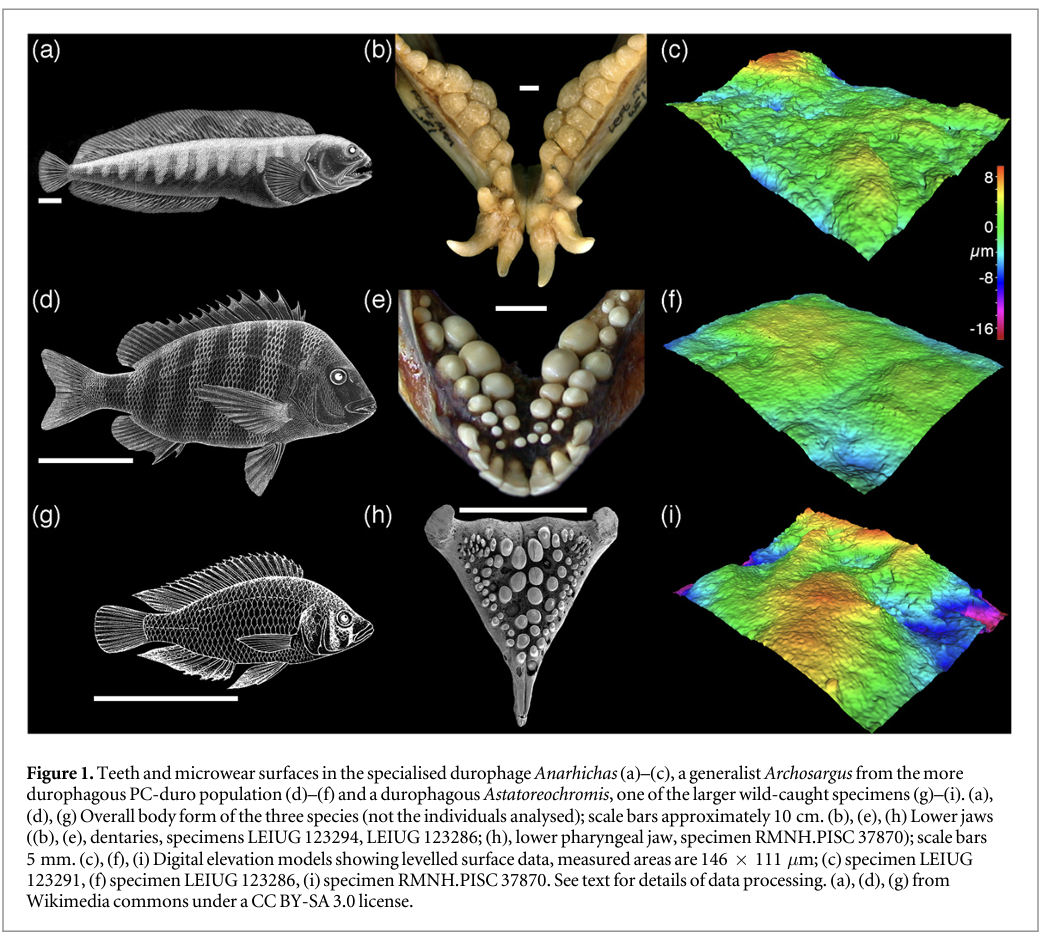
We sampled tooth microwear texture from six groups of fishes that differ in their diet (table 1), and environments (bathymetry, temperature, salinity). In all fishes, however, the functional surfaces of the jaws are composed of multiple molariform or bunodont teeth—a tooth shape that is typically interpreted as indicative of a durophagous diet in fossil fishes (e.g. Jose Poyato-Ariza and Didier Bermudez-Rochas 2009, Goatley et al 2010). Our samples include: two populations of sheepshead seabream, Archosargus probatocephalus (Teleostei, Sparidae, n = 6 individuals from each population; premaxillae sampled); one group of Atlantic wolffish, Anarhichas lupus (Teleostei, Anarhichadidae, n = 4 individuals; premaxillae and vomers sampled); two samples of wild-caught and one laboratory-reared Alluaud's haplo, Astatoreochromis alluaudi (Teleostei, Cichlidae, n = 3 for each sample; lower pharyngeal jaws). Figure 1 shows body form, tooth morphology and the nature of worn tooth surfaces in the three species studied.
The two populations of Archosargus are from the Indian River lagoon (previously studied by Cutwa and Turingan 2000) and were provided by Dr Ralph Turingan. One population (IR-herb) comes from the Southern part of the lagoon, the other (PC-duro) comes from the northern area, close to Port Canaveral. Both populations are dietary generalists but the IR-herb population consumes a significantly lower proportion of hard-shelled prey (such as bivalves) and a higher proportion of plant material (mean volumetric contributions: PC-duro—42.55% hard shelled prey, 18.31% plant material; IR-herb—24.58% hard shelled prey, most swallowed whole, 35.70% plant matter) (Cutwa and Turingan 2000, Turingan pers. comm. 2008). There are no clear morphological differences between populations (Cutwa and Turingan 2000).
The Anarhichas studied were collected from local processors in Aberdeen and sent to Leicester in 2005. Gut content data was not available but in natural conditions Anarhichas from the North Sea always incorporates a large proportion (circa 70% or more) of crushed invertebrates in its diet (Liao and Lucas 2000a, 2000b). The spawning season for wolffish is followed by the loss and replacement of the whole dentition (Liao and Lucas 2000b); the material available included individuals with heavily damaged tooth surfaces (assumed to be teeth that have accumulated wear for the greater part of the annual replacement cycle), and other individuals with teeth that are less worn, retaining a visual aspect similar to that seen on the dental surfaces of Archosargus. In order to ensure fair comparisons between populations (Archosargus and Astatoreochromis shed teeth more frequently than Anarhichas) only the less worn teeth are included in this analysis (for details see Darras 2012).
The studied lower pharyngeal jaws of Astatoreochromis come from three populations: one sample was laboratory-raised with a controlled, soft diet of minced heart and liver, with vitamins and Tetramin flakes (sample numbers RMNH.PISC 37864, 37865, 37866, 'laboratory'). The other two samples were captured in Mwanza Gulf and Kissenda Bay of Lake Victoria, and are separated based on their size compared to the laboratory sample: specimens RMNH.PISC 37870, 37871, and 37872 are similar in standard length but have larger lower pharyngeal jaws ('large wild'), while specimens RMNH.PISC 37867, 37868, and 37869 ('small wild') have lower pharyngeal jaws of similar dimensions but smaller standard length compared to the lab-raised fish. The same samples were used by Purnell et al (2012).
In some respects, our small sample sizes are not ideal, but importantly they allow us to test the discriminatory power of dental texture microwear analysis in situations where only a few specimens are available, and this is a real issue for many palaeodietary studies because of the scarcity of well-preserved fossil material. Furthermore, if our methods can provide reliable information from few individuals, this has the potential to reduce the impact on wild populations of sampling for dietary analysis.
2.2. Surface texture data acquisition
Because the translucency of enameloid creates difficulties for data capture using focus variation microscopy, surface data from Astatoreochromis were acquired directly from gold-coated teeth (Purnell et al 2012), and all other data were acquired from high fidelity surface replicas. These were prepared using Coltène-whaledent Speedex light body polyvinylsiloxane moulding compound, and EpoTek 320 LV black epoxy. Both were mixed and applied following the manufacturer's instructions. Analysis of accuracy and precision of moulding compounds indicates that replicas made this way compare favourably with the most accurate and precise moulding compounds, with very small absolute differences in parameter values between replica and original (Goodall et al 2015).
High-resolution 3D surface data were captured, following the methods of Purnell et al (Purnell et al 2012, 2013), with an Alicona Infinite Focus microscope G4b (IFM; Alicona GmbH, Graz, Austria; software version 2.1.2), using x100 objective to give a field of view of 146 × 111 μm. The Alicona Infinite Focus microscope G4b has a CCD of 1624 × 1232 pixels. In theory, for a field of view of 146 μm, this equates to a lateral sampling distance of 0.09 μm, but the limits imposed by the wavelength of white light mean that lateral optical resolution is actually about 0.35–0.4 μm. For all samples, vertical resolution was set at 20 nm, and the lateral resolution factor for the IFM was set at 0.3. Exposure and contrast settings were manually adjusted to maximise data quality. After manual deletion of defects, point clouds were exported as sur files and imported into SurfStand (software version 5.0). Surfaces were then automatically treated by levelling the surface and removing gross tooth form with a 2nd order polynomial function, and applying a robust spline filter, with a nesting index of 0.025 mm. The resulting scale limited roughness surface was then used for calculation of ISO 25178-2 standard parameters (ISO 25178-2 2012). More details of materials and techniques can be found in the supplementary material, including short definitions of ISO parameters (table S1).
2.3. Statistical analysis
For Archosargus analyses were based on a maximum of five samples (surface data collected from different teeth) per individual. Using multiple samples to a degree mitigates the effects of small sample numbers but there is a risk that assumptions of independence of observations are violated. In this case, however, the risk is small because the independence of texture data from samples within an individual is comparable to the independence of data from individuals within a population (based on pairwise comparisons of samples; see supplementary material): in both cases fewer than 50% of pairwise comparisons yield significant correlations. Furthermore, it is unlikely that our approach is significantly inflating the risk of type I errors (incorrectly rejecting the null hypothesis of no difference between populations) because analysis using a mean value for each individual yielded similar results to those presented below (see supplementary material).
For tests of the hypotheses that microwear texture differs between populations, between three and five samples per individual were used so that no single specimen would overweight the analysis, and to limit the risk of over-dispersion. Similar sampling was used for Anarhichas. Roughness parameters exhibiting non-normal distributions (Shapiro-Wilks test) were log-transformed; if they still deviated from normality they were rank-transformed. Rank-transformation does not provide normally distributed data but allows parametric testing of a power equivalent to that of non-parametric alternatives (Conover and Iman 1981, Zimmerman 2012). All analyses were performed with JMP 11 (SAS Institute, Cary, NC, USA).
Data were explored using t-tests, analysis of variance (ANOVA), correlations, pairwise testing (Tukey HSD and pairwise t-tests), principal components analysis (on correlations; PCA) and linear discriminant analyses (LDA). Where homogeneity of variance tests (Bartlett and Levene tests) revealed evidence of unequal variances, Welch ANOVA was used. The significance of LDA was assessed using Wilks' Lambda.
3. Results
3.1. Testing hypothesis 1: microwear texture does not differ between populations of Archosargus
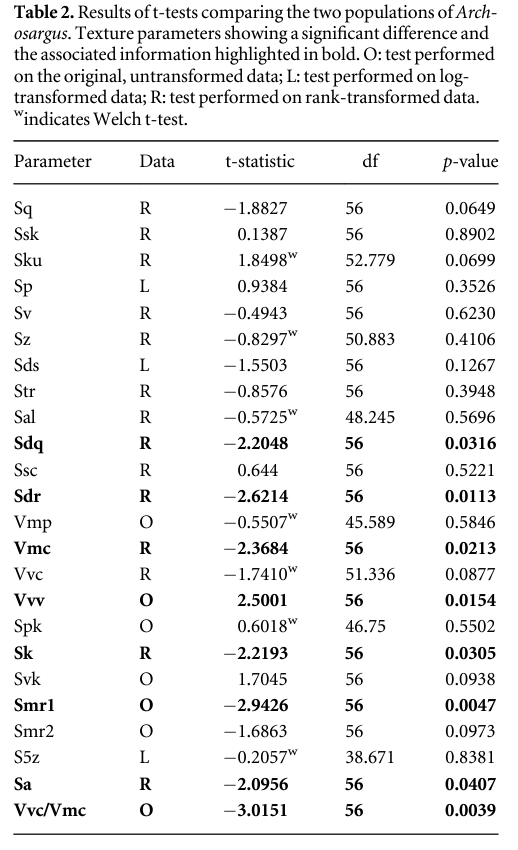
Comparing tooth surface textures in Archosargus from the IR-herb and PC-duro populations reveals that 8 ISO parameters differ significantly between populations (table 2). These are Sdq, Sdr, Vmc, Vvv, Sk, Smr1, Sa and Vvc/Vmc, most of which capture aspects of the aerial material ratio of the roughness surface—Sdq is the root mean square gradient of the surface; Sdr, the developed interfacial area ratio, is the percentage difference between the surface area of the texture compared to the cross sectional area of the surface; Vmc is volume of material making up the core of the surface; Vvv is the void volume of the valleys; Sk, core roughness depth, is the peak to valley depth of the surface with the predominant peaks and valleys removed; Smr1, the surface bearing area ratio, is the proportion of the surface which consists of peaks above the core material; Sa is the average height of surface; Vvc/Vmc is the ratio of the void volume to the material volume of the core of the surface (see table S1 for short definitions of all parameters). These results allow us to reject the null hypothesis that tooth microwear texture does not differ between two morphologically similar populations of a species that have different diets.
3.2. Testing hypothesis 3: microwear texture does not differ between specialist and opportunist shell crushers
Whether dental microtexture records qualitative (shell-crusher versus herbivore) or quantitative differences (proportion of crushed, hard-shelled prey in the diet) was tested by comparing the two populations of Archosargus with individuals of Anarhichas (with twice as much crushed, hard-shelled prey in its typical diet compared to the PC-duro population of Archosargus). Results indicate significant differences between trophic categories for the majority of textural parameters (table 3), with the few parameters that do not distinguish between the trophic categories (Ssk, Sds, Str, Sal, Smr2) also failing to show differences between the two populations of Archosargus (see above).
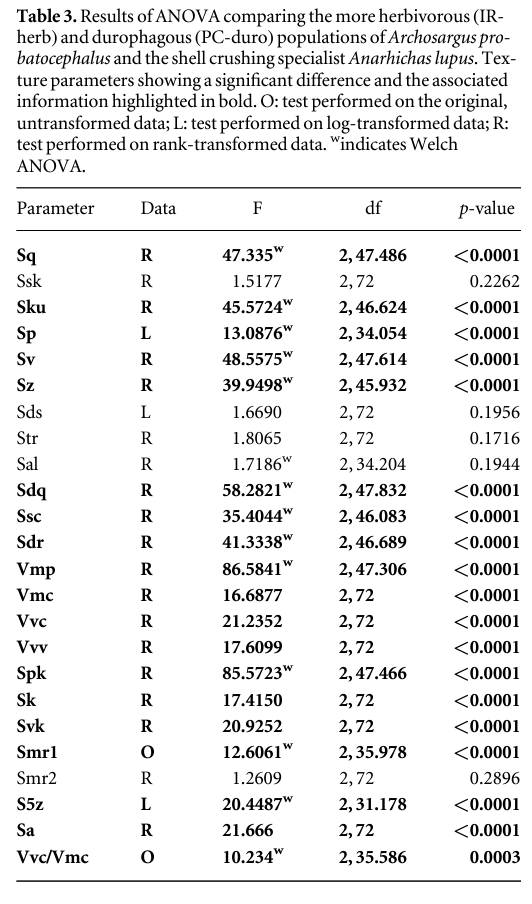
Pairwise t-tests reveal that nine parameters differ significantly (p < 0.05) between the three trophic categories: Sku, Sdq, Sdr, Vmc, Vvv, Sk, Smr1, Sa and Vvc/Vmc (except for Sku these are the same parameters that differ in the ANOVA of the two Archosargus populations). Of these, all but three parameters exhibit a trend of increase in value with increasing durophagy (lowest in Archosargus IR-Herb, and highest in Anarhichas; for Sku, Smr1 and Vvc/Vmc PC-duro have the lowest values). The more conservative HSD test finds fewer three way differences between the trophic categories (Sdr and Vvc/Vmc) but separates Anarhichas from the two Archosargus populations on all other parameters that differ. These results allow us to confidently reject the null hypothesis that microwear texture does not differ between specialist and opportunist shell crushers, with clear pairwise differences between trophic groups that correspond to the amount of shelly prey in their diet.
3.3. Multivariate models and cross taxon assignment to trophic groups
Principal components (PCA) and LDA were performed on the data from the three trophic groups of Archosargus and Anarhichas. Only the texture parameters that exhibit pairwise differences were included: Sku, Sdq, Sdr, Vmc, Vvv, Sk, Smr1, Sa and Vvc/Vmc. For the LDA three trophic categories were used—herbivorous generalist (IR-herb Archosargus), durophagous generalist (PC-duro Archosargus) and specialised durophage (Anarhichas).
PCA of these nine parameters (figure 2) reveals separation of trophic categories in a dietary space defined by PC axes 1 and 2, which together explain 85% of the variance. Sdq, Sdr, Vmc, Vvv, Sk and Sa load most heavily on the PC1 axis (0.87–0.95), while Sku (−0.55) and Smr1 and Vvc/Vmc weight onto PC2 (0.89, 0.88). Ranking fish populations from most (Anarhichas) to least durophagous (Archosargus IR-herb), PC1 is significantly correlated with diet (Rs = 0.62, P < 0.0001), and although there is a degree of overlap between categories, they occupy different areas of the plot (figure 2): all but one specialised durophage tooth (Anarhichas) has negative values for PC1, the durophagous generalists occupy the centre of the plot, with most teeth plotting between PC1 values of −1 and 2.5, and the herbivorous generalists plot mainly between 0 and 4. There is also a degree of separation along PC2, with about half the durophagous generalist Archosargus plotting below PC2 values of 0.1, whereas only two teeth from each of the other trophic categories plot in this area.
LDA produced a similar result to PCA analysis (figure 2), but with greater separation between trophic categories. The LDA is highly significant (Wilks' lambda = 0.237, p < 0.0001), with axis 1 accounting for 88% of variance. Overall, 79% of teeth were correctly assigned to trophic category based on the nine texture parameters, within category success ranging from 100% for specialised durophage (Anarhichas) to 72% for the two generalist (Archosargus) populations. Of the durophagous generalist teeth (PC-duro) 3% were incorrectly assigned to the specialised durophage category, and 24% to the herbivorous generalist; of the generalist herbivore teeth (IR-herb) 7% were incorrectly assigned to the specialised durophage category, and 21% to the durophagous generalist. Both canonical axes are correlated with the durophagy rank of the three populations (Axis 1 Rs = 0.46, p < 0.0001; Axis 2 Rs = 0.37, p = 0.0012).
The multivariate analyses provide strong evidence that tooth microwear texture can differentiate between fish populations that vary in the proportions of shelly prey they consume, but we further tested the degree to which the results can be extrapolated beyond the three populations studied by applying the discriminant functions derived from the LDA to teeth from Astatoreochromis alluaudi. Such a test gives an indication of whether tooth microwear texture could be used to reliably determine the diet of an extinct fish with apparently durophagous jaw and tooth morphology, except we are able to compare the results with the known diet of the Astatoreochromis. This is a stringent test, as texture parameter data from the Astatoreochromis teeth played no part in structuring the LDA.
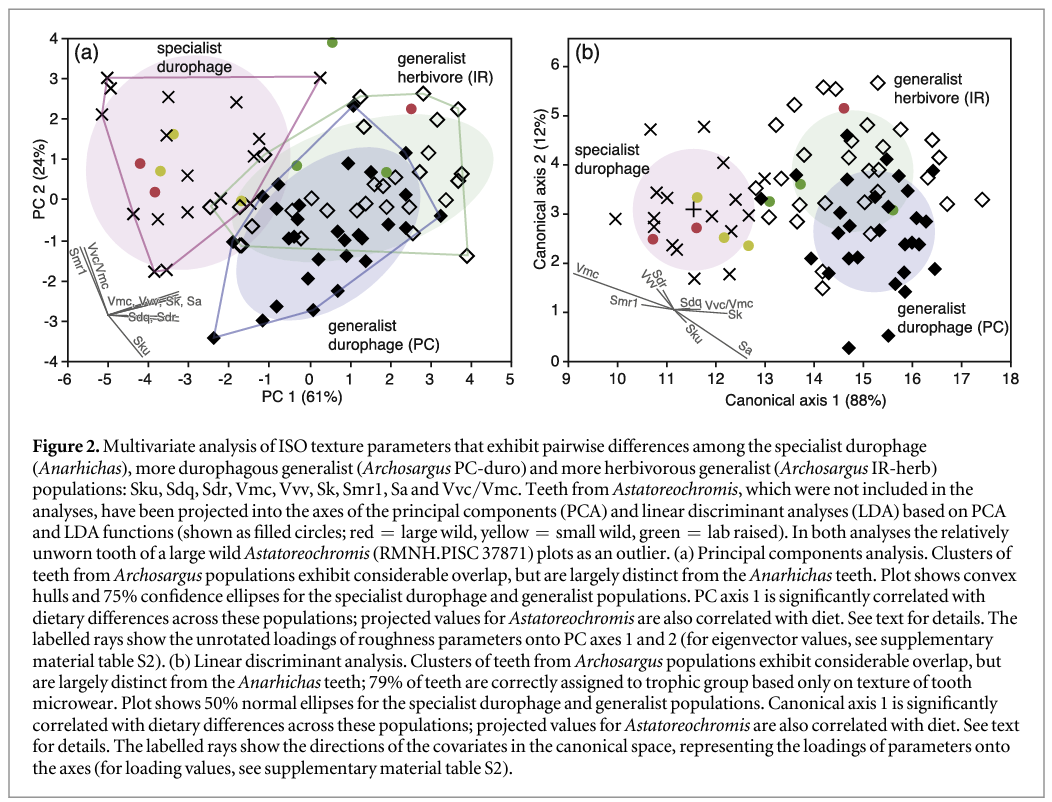
Visual inspection of the distribution of the Astatoreochromis teeth show that all but one of the teeth from wild caught, durophagous individuals plot among the data for the specialised durophage while the teeth from lab raised individuals fed a soft diet plot among the generalist Archosargus. Anomalously, one tooth from the large wild caught fish plots among the generalist herbivores, well away from all the other teeth from wild caught fish. This tooth (RMNH.PISC 37871) was also found to plot towards teeth from lab-raised fish in the PCA of Purnell et al (2012), and in qualitative visual assessment of relative roughness of two-dimensional images this tooth was consistently identified as being among the least worn in the study (Purnell et al 2012). It thus seems likely that the tooth was relatively newly erupted at the time the fish was collected, and we exclude it as an outlier from subsequent analysis. The remaining teeth from the large wild caught fish were assigned by the discriminant function to the specialised durophage category (with >99% probability); the teeth from small wild fish were also assigned to this category (85%–99% probability). Of the three lab-raised fish, none is unequivocally assigned to the specialised durophage category. One is assigned to this group with a marginal probability of only 51%; the other two are assigned to generalist categories (1 to each, probabilities of 66% for the generalist herbivore assignment, 62% for the generalist durophage assignment, with 38% probability of assignment to the more herbivorous group). Of particular note is that the known diet of the Astatoreochromis correlates significantly with the first axis of the LDA (Rs = −0.941, p = 0.005 for diet ranked according to the amount of hard-shelled prey in stomach/gut contents; Rs = −0.945, p = 0.0004 for diet ranked 1–3 from wild large to wild small to lab-raised soft food).
Projecting the Astatoreochromis texture data into the PCA analysis of Anarhichas and Archosargus gives similar results. The teeth from wild caught fish plot among the specialist durophage data (except for RMNH.PISC 37871). The teeth from lab-raised fish plot among, or close to, the generalist herbivore data. PC axis 1 correlates strongly with diet (Rs = −0.941, p = 0.005 for diet ranked according to the amount of hard-shelled prey in stomach/gut contents; Rs = −0.945, p = 0.0004 for diet ranked 1–3 from wild large, to wild small, to lab-raised soft food).
4. Discussion
The results presented here show that analysis of the microwear texture of molariform teeth in fishes can track subtle dietary differences between populations of conspecific individuals. It can also highlight differences within a group of individuals. For analysis of Archosargus, the morphologically identical populations of fishes studied by Cutwa and Turingan (2000) differed mostly in the proportions of hard prey consumed, but the technique the authors used for dietary analysis (gut content analysis) provides only a 'snapshot' of the diet in the few hours or perhaps days prior to the capture of the animal and will likely provide an estimate of what was available in the environment where and when an individual fed last. Observed variability in diet within a species can be seasonal (Pallaoro et al 2006, Fehri-Bedoui et al 2009) as well as geographic (Mariani et al 2002, Langerhans et al 2003) or an interaction of these two factors (e.g. Castillo-Rivera et al 2007, Chuwen et al 2007) but also simply the result of a sampling bias depending on the technique used. Analysis can also be hampered by a large number of individuals having empty stomachs. In contrast to stomach contents analysis, dental microwear accumulates over a period of days or weeks. This avoids the snapshot bias of stomach contents analysis (Purnell et al 2012), but the data remain sensitive enough to track shifts in diet over time, or seasonal patterns (Estebaranz et al 2009, Merceron et al 2010). If diet changes significantly there is a lag time equivalent to the time it takes for microwear generated by the new diet to replace that reflecting the old diet, but experimental analysis of stickleback teeth suggests that in fishes this lag time can be as short as 4 days (Baines et al 2014).
Interspecific comparison highlights the large number of differences in microwear texture between Archosargus and Anarhichas, reflecting the fact that proportion of hard shelled prey in the Anarhichas diet is twice that of the most durophagous population of Archosargus. This analysis also confirms that differences in microwear observed between populations of Archosargus are quantitative (linked to the proportion of hard shelled prey in the diet) rather than qualitative (linked to the occurrence of crushing). The significant correlations between diet and the multivariate axes derived from analysis of Anarhichas and Archosargus provide further compelling support. Furthermore, despite the differences in size and habitat between these taxa and Astatoreochromis, the multivariate models derived from microwear texture data alone plotted most of the Astatoreochromis teeth in the same region of PCA and LDA space as the dietary categories that were closest to their actual diets. The significant correlations of the actual diet of Astatoreochromis with the values for PC1 and canonical axis 1 of the LDA predicted from the analyses—a particularly stringent test—gives a clear indication of the potential power of the approach for taxon independent analysis of diet in fishes.
The multivariate analyses demonstrate that dental microwear texture analysis is an effective tool to separate populations of fishes based on the proportion of hard prey they process. However, although the ANOVA finds significant differences between the microwear textures of the two populations of Archosargus, there is a degree of overlap in microwear between teeth from the more durophagous and the more herbivorous populations. Even though the LDA correctly assigns 72% of the Archosargus teeth to trophic group on the basis of microwear texture alone, this overlap is also evident in the multivariate analysis. This is not surprising. Stomach content data for the Archosargus individuals sampled for microwear were not available, but Cutwa and Turingan (2000) documented variability within populations in the proportion of hard shelled prey and plant matter consumed, and it seems likely that the overlap between the microwear is at least in part a reflection of an overlap in dietary composition in the wild. A further confounding effect comes from the fact that texture samples from within an individual will reflect different periods of tooth use since eruption (teeth are not all shed and replaced at the same time), creating additional non-dietary noise in the microwear signal.
Few previous analyses have used ISO parameters to investigate the relationship between the texture of tooth microwear and diet, but some consistent patterns are starting to emerge. Of the parameters that differ between populations in the analysis presented here, Purnell et al (2013) found that Vmc, Vvv and Sk were significantly correlated with diet and increased with increasing 'hard' prey in the diet, as they are in our analysis (see Purnell et al 2013, for discussion of 'hard' versus 'soft' 'food'). Sa, in contrast to our results, they found to decrease with the amount of 'hard' prey in the diet of bats. Schulz et al (2013) also found Vmc and Vvv, and Sa, to increase with what they interpreted to be more abrasive diets in grazing ungulate mammals. Direct comparisons of parameters with Purnell et al (2012) is difficult because they used a different approach to the generation of scale limited surfaces, but the lower pharyngeal jaws of Astatoreochromis in their analysis are the same as those analysed here, and our analysis indicates that most of the parameters that differ between populations of Archosargus and Anarhichas increase with the amount of hard shelled food in the diet of the cichlids (Vmc, Vvv, Sk, Sdq, Sdr).
In terms of the ecological, environmental, and animal size range across which textural analysis of microwear is applicable, our analysis provides the broadest test yet conducted. Astatoreochromis alluaudi is found in a variety of freshwater settings in Africa, including lakes and rivers with different degrees of turbidity, oxygenation, etc (Binning and Chapman 2010, Binning et al 2010). Both biotic (e.g. abundance and type of food) and abiotic (temperature, depth, salinity) aspects of these environments differ from those found in the Florida lagoons from which Archosargus were obtained, and the rocky marine environments of the north Atlantic where the Anarhichas were captured. The species analysed differ significantly in size (common TL for Archosargus = 350 mm; max length for Anarhichas = 1500 mm; max length for Astatoreochromi = 190 mm), and differences in size could influence microwear texture if gape size leads to consumption of a type of prey that induces a significantly different mechanical stress on the dental surfaces. Teeth analysed also differed in their location in the oropharyngeal cavity: Astatoreochromis teeth were sampled from the pharyngeal jaws while others were sampled from the oral jaws and vomers. Despite all these potentially confounding factors, and the phylogenetic distance between the taxa involved, our analysis of dental microwear texture correctly discriminated between teeth from individuals with different diets and assigned most teeth to their correct trophic groups. Our analysis thus provides the foundations upon which to base future analyses of diet in extinct fishes and test hypotheses of durophagy that, at present, are based on the hypothesis that fish with a molarifom or bunodont 'crushing dentition' ate hard shelled prey. Clearly, this hypothesis is an oversimplification, and we predict that supposedly durophagous fishes had more complex ecological roles than has previously been thought.
5. Conclusions
Our results confirm that textural analysis of tooth microwear provides a powerful tool for analysis of the realised rather than the biomechanically possible diet of an organism, and that the approach is applicable to fishes. Dental microwear texture provides significant results even on small samples and animals with no gut content, and thus offers a potential means to reduce the impact on wild fish populations of analysing their dietary ecology. Moreover, our analysis suggests that models based on wild-caught populations can be used to infer diet in other taxa, even where they differ in habitat and body size. This shows that dental microwear texture analysis applied to extinct fishes has the potential to provide a robust new approach to testing of hypotheses of trophic ecology, niche segregation and escalation in jawed vertebrates through almost 400 million years of fossil record.
Acknowledgments
The authors would like to thank Dr Ralph Turingan from the Florida Institute of Technology for providing the samples of Archosargus, Dr David Baines for access to the samples of Anarhichas and Rob Goodall for comments on the ms. Funded in part by Natural Environment Research Council grants NE/B000125/1 and NE/G018189/1.
Author Contributions
MAP conceived the study and collected microwear data for Astatoreochromis. LPGD collected the microwear data for Archosargus and Anarhichas. Both authors designed the study, conducted the analysis, interpreted the results and wrote the paper.





