Assessing the importance of human activities for the establishment of the invasive Poa annua in Antarctica


Published: June 23, 2014
Latest article update: Aug. 21, 2023
Abstract
Because of its harsh environmental conditions and remoteness, Antarctica is often considered to be at low risk of plant invasion. However, an increasing number of reports have shown the presence and spread of non-native plants in Antarctica; it is therefore important to study which factors control the invasion process in this ecosystem. Here, we assessed the role of different human activities on the presence and abundance of the invasive Poa annua. In addition, we performed a reciprocal transplant experiment in the field, and a manipulative experiment of germination with P. annua and the natives Colobanthus quitensis and Deschampsia antarctica, in order to unravel the effects of physical soil disturbance on the establishment and survival of P. annua. We found a positive correlation between abundance of P. annua and level of soil disturbance, and that survival of P. annua was 33% higher in sites with disturbed soil than non-disturbed. Finally, we found that disturbance conditions increased germination for P. annua, whereas for native species germination in experimentally disturbed soil was either unchanged or reduced compared to undisturbed soil. Our results indicate that human activities that modify abiotic soil characteristics could play an important role in the abundance of this invasive species. If the current patterns of human activities are maintained in Antarctica, the establishment success and spread of P. annua could increase, negatively affecting native flora.
Keywords
Colobanthus quitensis, Deschampsia antarctica, Alien species, human disturbance, Poa annua, tourists
Considered one of the harshest environments worldwide, Antarctica is characterized by low temperatures, high solar radiation, strong winds, a short growing season and limited water and nutrient availability (Robinson et al. 2003; Wasley et al. 2006). Antarctica has been considered less susceptible to biological invasions than other ecosystems due to its severe environmental conditions, isolation and exceptionally low species diversity (Convey 2003; Hughes et al. 2010). Nevertheless, a number of studies have documented the presence of alien species in maritime Antarctica and on the mainland (Lewis-Smith 1996; Frenot et al. 2005; Chwedorzewska 2008). Many of the alien species reported in these studies are low in abundance and are restricted to disturbed sites (Chwedorzewska 2008, 2009; Chwedorzewska & Korczak 2010; Molina-Montenegro et al. 2012; Cuba-Díaz et al. 2013). The non-native species are mainly found in the vicinity of scientific bases and tourist landing areas, suggesting that their presence and abundance are facilitated by human activities (Chwedorzewska & Korczak 2010; Chown et al. 2012; Molina-Montenegro et al. 2012).

Human travel is occurring at an unprecedented level across the globe and is becoming a major vector for the transfer of propagules, such as seeds, eggs and spores, of species alien to the Antarctica (e.g., Frenot et al. 2005; Lityńska-Zając et al. 2012). Introduction routes of alien organisms are largely associated with the movement of people and cargo from scientific programmes and to a lesser extent tourist operations (Frenot et al. 2005; Tin et al. 2009; Chown et al. 2012; Molina-Montenegro et al. 2012). All cargo, personal luggage, clothes and equipment of people visiting Antarctic stations can be a potentially contaminated by alien propagules. Several studies have examined the relationship between the number of alien species established in sub-Antarctica and Antarctica and the number of human visitors (e.g., Chown et al. 1998; Lee & Chown 2009a, 2009b). In spite of the constant increase in tourist visits mainly in the Antarctic Peninsula region, in many ways research stations could be considered to have a greater impact on the terrestrial environment. About 40 000 people, mostly tourists, visit the Antarctic coast every year, whereas Antarctica has a summer population of about 4000 researchers from different countries (Frenot et al. 2005; IAATO 2013). Scientific expeditions remain in one place much longer than tourists, affecting the environment around the station continuously, and bring in large quantities of supplies and equipment (which may be contaminated with soil and propagules). Scientific activity is most intense during summer melts, when vegetation is developing and seabirds and mammals are reproducing (e.g., Chwedorzewska & Korczak 2010; Chown et al. 2012). The construction of buildings and other infrastructure at Antarctic stations disturbs the soil, physically and chemically, creating areas around each station that could be particularly vulnerable to human impact (Chwedorzewska & Korczak 2010). Keeping stations supplied and maintaining infrastructure require the use of heavy vehicles, typically concentrated in a very limited area, which contributes to potential soil damage. Most stations are built in ice-free areas, which have the most favourable microclimates for the establishment of plants in the Antarctic.
Recent reviews have identified several pivotal factors in determining the establishment and spread of non-native invasive species (e.g., Richardson et al. 2000; Dietz & Edwards 2006; Theoharides & Dukes 2007). For instance, while the native community and abiotic features of the invaded habitat are important barriers for invasion (Richardson et al. 2000), disturbance is considered one of the main drivers of plant invasions (Hobbs & Humphries 1995; Londsale 1999). In Antarctic habitats, disturbances can be caused by natural geomorphological processes such as landslides, cryogenic soil movement (Convey 1996), by deglaciation and by animals, such as seals (Scott & Kirkpatrick 2005; Hausmann et al. 2013). The majority of non-native species reported in the Antarctica has been found in disturbed sites surrounding scientific bases (Olech 1996; Chwedorzewska & Korczak 2010; Molina-Montenegro et al. 2012; Cuba-Díaz et al. 2013) or in pioneer zones (Olech & Chwedorzewska 2011). Nevertheless, the establishment of alien vascular plants has recently also been reported in natural habitats (Olech & Chwedorzewska 2011).
One of the most ubiquitous plant species in the world, Poa annua is widely established in the sub-Antarctic and maritime Antarctica (see Hughes et al. 2010). The wide distribution of P. annua may result from its dispersal mechanism and physiological tolerance, mainly in areas subject to human activities or in pioneer zones (Frenot et al. 1997) and in those with disturbance and manuring from animal aggregations (Walton 1975). This alien species is considerate an invasive species in Antarctica, with negative impacts on the biomass and physiological performance of native vascular species, with which it competes for resources and/or space (Molina-Montenegro et al. 2012). In this study we assess different variables related to human activities with the abundance and the successful establishment of the invasive plant species P. annua in the Antarctic Peninsula, and South Shetland Islands. We also analyse the survival percentage of P. annua growing in sites with different kinds of soil disturbance. Finally, we evaluate the germination percentage of the alien P. annua and the native plants Colobanthus quitensis and Deschampsia antarctica subjected experimentally to different levels of soil disturbance.
Materials and methods
We visited 25 different sites on Antarctic islands and the Antarctic Peninsula during the Chilean Scientific Antarctic Expeditions of 2007/08 and 2009/10—the austral summer seasons—in order to search for populations of Poa annua (see Molina-Montenegro et al. 2012). In each site we visually surveyed the presence of P. annua around every building and point of landing (Fig. 1). Each plant individual was labelled in order to avoid recording the same individuals twice, and its distance to the closest buildings was recorded. Out of all sites visited we found individuals of P. annua at six of them. The plant was recorded at one point at each of five sites, whereas at the Polish Arctowski Antarctic Station it was recorded at two points.
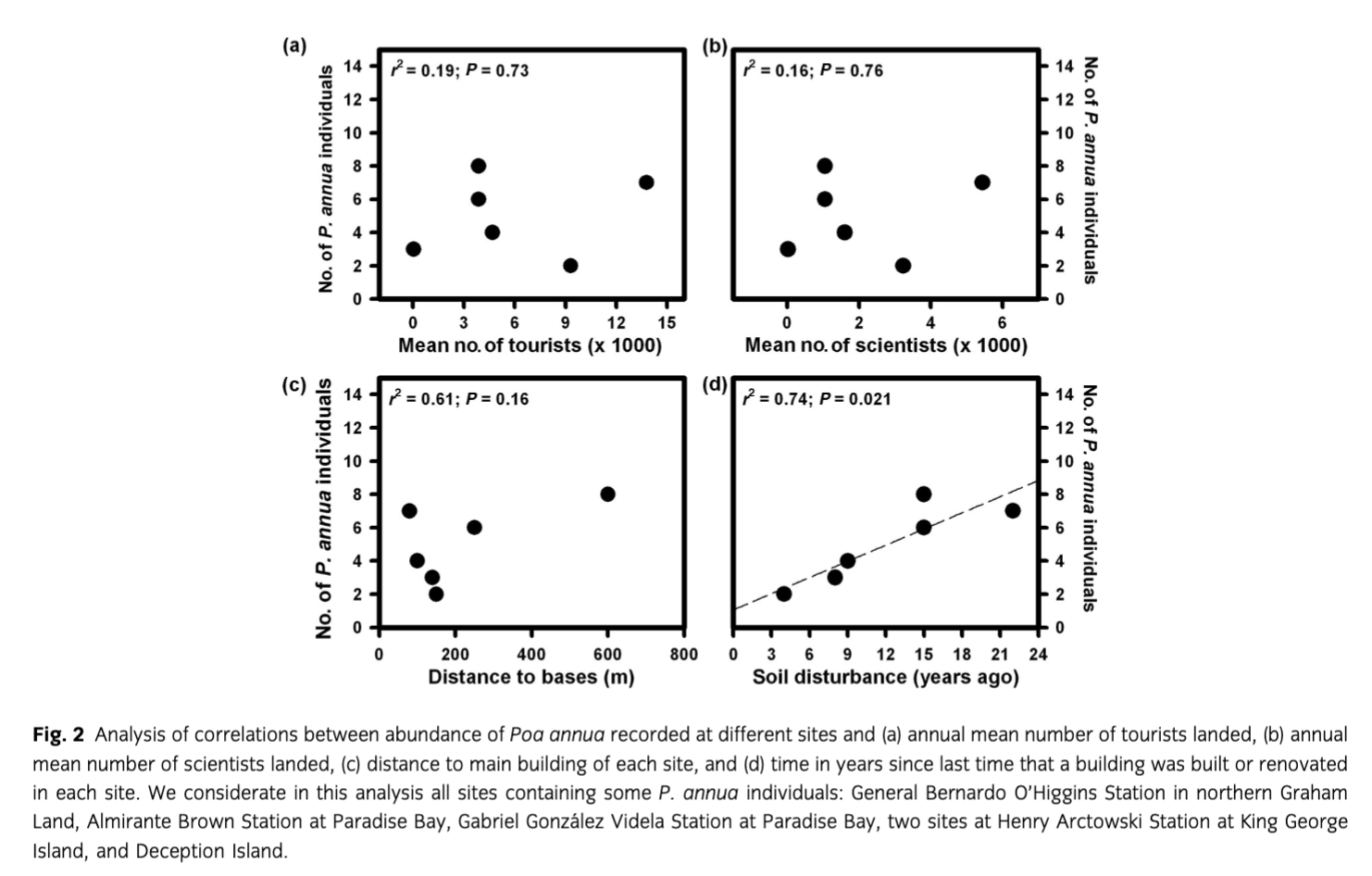
In order to find potential drivers of P. annua establishment, we performed a regression analysis using the abundance of this species and (i) mean number of tourists landed in each point where P. annua has been recorded, (ii) mean number of scientists and operators landed in each point where P. annua has been recorded, (iii) distance to the main building of each station and (iv) level of soil disturbance. We used the time in years from the last time when any building was constructed or renovated at each station as a proxy for soil disturbance. Drawing from the database of the International Association of Antarctica Tour Operators, we considered the mean number of visits (tourists and non-tourists) landed during the 2007/08 and 2009/10 summer seasons—the growing seasons that coincided with our fieldwork.
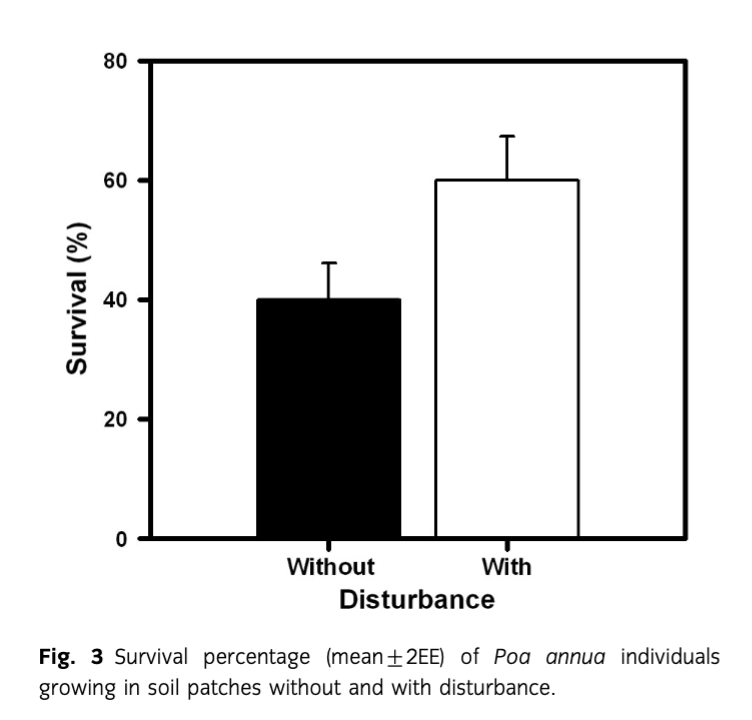
To assess the effect of different soil-patch types on establishment, we performed a transplant experiment with adult individuals of P. annua collected close to the coastline (within 30–40 m to the sea). This experiment was conducted in a study area near Arctowski Station. All plants used ranged in height from 5 to 7 cm and appeared healthy. Each individual plant was excavated together with the soil around the roots (ca. 500 g) and kept well-watered in a plastic box under natural conditions of light and temperature for 12 h until the transplant. Plant status was visually assessed just before the transplant (plants showing foliar and/or root damage were excluded). We considered two soil-patch types for the experiment; the first type was in the bare ground between the resident vegetation (natural) and the second was in the disturbed soil surrounding the scientific stations. We selected three patches by each soil-type, with distances of 23, 41 and 56 m among each pair of sites (natural–disturbed). At each patch, 10 P. annua individuals were planted within each of three soil-patches of each type (n=30 individuals per soil-type). Individuals were planted at least 5 cm apart from each other. Transplants were carried out during the 2010/11 growing season and seedling survival was evaluated after six weeks. Additionally, we compared nitrogen availability and soil moisture between five non-disturbed and five disturbed patches. A soil sample of 10 cm depth (ca. 100 g) was taken in each patch. This depth was selected is this the average depth of the root zone of P. annua in the study. Soil samples were stored in sealed plastic bags and sent for analyses to determine the concentration of nitrate (; Robarge et al. 1983) and ammonium (; Longeri et al. 1979). Soil moisture (soil matric potential) was measured in five non-disturbed patches and five disturbed patches during the mid-growth season (January 2010). At each sampling point, a 2725 series Jet Fill Tensiometer (Soil Moisture Equipment Corp., Santa Barbara, CA, US) was dug into the soil to a depth of 10 cm. Tensiometers were placed at mid-day and, after a stabilization period of 2 h, the soil matric potential was recorded. At the end of experiment, all surviving individuals were removed from the site and destroyed.
Finally, we performed a manipulative experiment to assess the effect of soil disturbance on germination of Pou annua, Colobanthus quitensis and Deschampsia antarctica. We collected seeds from the inflorescence of the previous year’s adult healthy-looking plants growing in tussocks in ice-free zones close to the coastline. At the commencement of this experiment three seeds of each species were tested to assess viability. This was done by cutting the seed in half on the equatorial axis with a razor blade after 24 h of immersion in tetrazolium chloride. All seeds were viable.
Each experimental unit was a plastic pot (50×40×30 cm) filled with soil extracted from the study site. Each experimental unit consisted 10 seeds of P. annua or C. quitensis or D. antarctica (n=6 pots per species; total n=18 pots). Seeds were buried 4–5 cm deep in a plastic pot, as this was the maximum depth at which P. annua seeds were found at the study site. Pots were subjected to a 20/4 h light/dark photoperiod at 4°C and were watered with 100 ml of water every four days. To determine the effect of soil disturbance on germination, half the pots of each species was subjected to manual disturbance in the top 4–5 cm of soil every three days until emergence of the first seedling (total n=9 pots). After the first germination, manual disturbance ceased in order to avoid seedling damage, but the recording of germination continued. The remaining pots—the controls—were undisturbed. Disturbance was deemed similar in intensity and effects to that of footfall and light vehicle traffic. At the conclusion of the experiment, which ran for three months, we recorded the number of emerged seedlings.
Statistical analysis
To investigate the relationship between the abundance of P. annua individuals and the different human activities in the South Shetland Islands and the Antarctic Peninsula, we used standard Pearson correlations. A one-way ANOVA was performed to compare the nitrogen content, water availability and survival percentage of P. annua individuals growing in soil patches with different disturbance type. To evaluate the effect of disturbance on the germination percentage in both natives and alien plant species, we conducted a two-way ANOVA. For all two-way ANOVAs, the assumptions of normality and homogeneity of variances were tested using the Shapiro–Wilks and Bartlett tests, respectively (Zar 1999).
Results
During the 2007/08 and 2009/10 growing season, the presence and abundance of P. annua at six different sites was recorded out of a total of 25 sites in the South Shetland Islands (one site at Deception Island and two sites at Arctowski Station) and the Antarctic Peninsula (Bernardo O’Higgins Station, Gabriel González Videla Station, and Almirante Brown Station). Regression analysis did not show a relation between P. annua abundance and the mean number of tourists or non-tourists landed at each scientific station (Fig. 2a, b). However, the distance of P. annua individuals to the main station building showed a positive but not significant correlation (Fig. 2c), and the abundance of P. annua was positively and significantly correlated with the number of years elapsed since any building was constructed or renovated (Fig. 2d).
In the transplant experiment, the survivorship of P. annua individuals was significantly higher (F1, 8=6.11; P=0.039) in sites with soil disturbance than without disturbance (Fig. 3). Soil moisture was not different (F1, 8=28.52; P=0.09) between disturbed and non-disturbed patches of soil (−25±4 and −30±6 MPa disturbed and non-disturbed, respectively). In contrast, the available nitrogen content of the disturbed soil was significantly higher (F1, 8=231.21; P<0.01) than in non-disturbed conditions (7.56±1.2 and 3.98±1.6 mg·K−1 disturbed and non-disturbed, respectively).
Overall, the percentage of germination was significantly increased under the disturbance treatment (F1, 24=563.33; P<0.001), but this trend was not similar for all species ( F2, 24=364.25; P<0.001). For P. annua the germination increased with disturbance, for C. quitensis germination decreased significantly under disturbance while for D. antarctica the germination percentage was not different between the disturbance and control treatments (Fig. 4).

Discussion
In the 21st century, the number of visitors to the Antarctica and the scale of human influence have increased dramatically, mainly due to tourism (IAATO 2013). There has been concern about the potential impact of visitors on the Antarctic environment (Culik & Wilson 1995; Chown et al. 2012). Several studies have aimed to detect the effects of this visitor pressure (Chwedorzewska & Korczak 2010).
We did not find a positive correlation between Poa annua abundance and the mean number of tourists or scientists visiting the Antarctic. However, a positive relationship between the abundance of P. annua and the time since a base underwent construction suggests that this kind of human activity influences the establishment phase of this species. We believe that human activity associated with research stations enhances soil nutrients and modifies the soil matrix, and therefore facilitates the germination and establishment of P. annua. Several studies have shown that cargo and vehicles transported to Antarctic research stations contain propagules of many plants (Whinam et al. 2005; Lee & Chown 2009b; Chown et al. 2012). Hughes et al. (2010) highlighted that research station construction also facilitates the transport of non-native seeds. We found that time since construction influences the presence of P. annua, most likely because more time allows for the establishment and population expansion of the plant. In Antarctica, ice-free areas near the coast are rather scarce and are hotspots for local fauna and flora (Convey 1996) as well as humans (Chown et al. 2012). High traffic around research stations creates a high level of soil disturbance, which can facilitate the establishment of exotic plants (Chwedorzewska 2008; Cuba-Díaz et al. 2013), as our experiments support. In stressful environments such as those found in the Antarctic terrestrial ecosystems, it has been predicted that alien species are unlikely to colonize and extend their range of distribution unless some process relaxes the environmental constraints (Dullinger et al. 2003; Pauchard et al. 2009).
It is widely assumed that soil disturbance enhances resource availability (e.g., Burke & Grime 1996; Davis et al. 2000), and some have examined the effect of disturbances in cold environments and shown increased nutrients, or higher accessibility, following disturbance (Stanton et al. 1994; Grieve 2000). We have elsewhere shown that alien species take advantage of high resource availability (e.g., nutrient or water), improving their ecophysiological performance (Molina-Montenegro et al. 2011). In the study reported here, we found that disturbed soils had higher levels of nutrients, as well as germination and survival percentage of P. annua, suggesting that disturbance by humans may generate microhabitats more favourable to P. annua than to native plants. Alternatively, the enhanced establishment and survival of P. annua in disturbed areas could result from non-random spatial distribution due to wind. In cold environments some plant species show greater establishment and survival percentages when sheltered from the desiccating effect of wind (Resler et al. 2005). In Antarctica P. annua has been shown to become established at sites sheltered from wind, such as alongside buildings (Olech 1996). More surveys should be conducted to further explain the spatial distribution and performance of P. annua in Antarctica.
Since it was first recorded in the mid-1980s (Olech 1996), P. annua is the only introduced plant species to have established permanent scattered populations in the Shetland Islands and on the Antarctic Peninsula (Frenot et al. 2005; Chwedorzewska 2008, 2009; Molina-Montenegro et al. 2012). The species has shown a steady, constant increase in abundance and number of new populations (Molina-Montenegro et al. 2012). Having spread to alpine ecosystems (Johnston & Pickering 2001), northern Europe (Gederaas et al. 2012) and Sub-Antarctic islands (Frenot et al. 2005), P. annua is “generalist” invasive species that tolerates stressful environments and takes advantage of favourable conditions, e.g., enhanced nitrogen availability in disturbed soils.
Surprisingly, nowadays there is no international policy to control the introduction of non-native species to Antarctica. Our study provides evidence that Antarctic environments could be severely impacted in the near future by such introductions and in the long-term they may have unsuspected consequences on Antarctic diversity. Although, some efforts have been made to accomplish this goal, they are still insufficient to mitigate the negative impacts of human activities on Antarctic biodiversity (see Hughes & Convey 2014).
In summary, P. annua is positively correlated with human activities and its survival and germination is enhanced in soil disturbance conditions. We conclude that human impacts, mainly associated with research activities, may have negative effects on the native species but positively influence the abundance and spread of the invasive P. annua on the Antarctic continent.
Acknowledgements
We acknowledge the financial support of the Chilean Antarctic Institute (INACH project T-14-08 and G-22-11).
References
- Burke M.J.K. & Grime J. 1996. An experimental study of plant community invisibility. Ecology 77, 776–790. Publisher Full Text
- Chown S.L., Gremmen N.J.M. & Gaston K.J. 1998. Ecological biogeography of Southern islands: species–area relationships, human impacts and conservation. American Naturalist 152, 562–575. PubMed Abstract | Publisher Full Text
- Chown S.L, Huiskes A.H.L., Gremmenb N.J.M., Lee J.E., Teraudsa A., Crosbie K., Frenot Y., Hughes K.A., Imura S., Kiefer K., Lebouvier M., Raymond B., Tsujimoto M., Ware C., Van de Vijver B. & Bergstrom D.M. 2012. Continent-wide risk assessment for the establishment of nonindigenous species in Antarctica. Proceedings of the National Academy of Science of the United States of America 109, 4938–4943. Publisher Full Text
- Chwedorzewska K.J. 2008. Poa annua L. in Antarctic: searching for the resource of introduction. Polar Biology 31, 263–268. Publisher Full Text
- Chwedorzewska K.J. 2009. Terrestrial Antarctic ecosystem at the changing world—an overview. Polish Polar Research 30, 263–273. Publisher Full Text
- Chwedorzewska K.J. & Korczak M. 2010. Human impact upon the environment in the vicinity of Arctowski Station, King George Island, Antarctica. Polish Polar Research 31, 45–60. Publisher Full Text
- Convey P. 1996. The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biological Reviews of the Cambridge Philosophical Society 71, 191–225. Publisher Full Text
- Convey P. 2003. Maritime Antarctic climate change: signals from terrestrial biology. Antarctic Research Series 79, 145–158.
- Cuba-Díaz M., Troncoso J.M., Cordero C., Finot V.L. & Rondanelli-Reyes M. 2013. Juncus bufonius, a new non-native vascular plant in King George Island, South Shetland Islands. Antarctic Science 25, 385–386. Publisher Full Text
- Culik B.M. & Wilson R.P. 1995. Penguins disturbed by tourists. Nature 376, 301. Publisher Full Text
- Davis M.A., Grime J.P. & Thompson K. 2000. Fluctuating resources in plant communities: a general theory of invisibility. Journal of Ecology 88, 528–534. Publisher Full Text
- Dietz H. & Edwards P.J. 2006. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87, 1359–1367.
- Dullinger S., Dirnböck T. & Grabherr C. 2003. Patterns of shrub invasion into high mountain grasslands of the northern calcareous Alps, Austria. Arctic, Antarctic and Alpine Research 35, 434–441.
- Frenot Y., Chown S.L., Whinam J., Selkirk P.M., Convey P., Skotnicki M. & Bergstrom D.M. 2005. Biological invasions in the Antarctic: extent, impacts and implications. Biological Review 80, 45–72. Publisher Full Text
- Frenot Y., Gloaguen J.C. & Trehen P. 1997. Climate change in Kergulen Islands and colonization of recently deglaciated areas by Poa kergulensis and P. annua. In B. Battaglia (ed.): Antarctic communities: species structure and survival. Pp. 358–366. Cambridge: Cambridge University Press.
- Gederaas L., Moen T.L., Skjelseth S. & Larsen L-K. 2012. Alien species in Norway—with the Norwegian Black List. Trondheim: Norwegian Biodiversity Information Centre.
- Grieve I.C. 2000. Effects of human disturbance and cryoturbations on soil iron and organic matter distributions and on carbon storage at high elevations in the Cairngorm Mountains, Scotland. Geoderma 95, 1–14. Publisher Full Text
- Hausmann N.S., Rudolph E.M., Kalwij J.M. & McIntyred T. 2013. Fur seal populations facilitate establishment of exotic vascular plants. Biological Conservation 162, 33–40. Publisher Full Text
- Hobbs R.J. & Humphries S.E. 1995. An integrated approach to the ecology and management of plant invasions. Conservation Biology 9, 761–770. Publisher Full Text
- Hughes K.A. & Convey P. 2014. Alien invasions in Antarctica—is anyone liable? Polar Research 33, article no. 22103, doi: 10.3402/polar.v33.22103. Publisher Full Text
- Hughes K.A., Convey P., Maslen N.R. & Smith R.L.I. 2010. Accidental transfer of non-native soil organisms into Antarctica on construction vehicles. Biological Invasions 12, 875–891. Publisher Full Text
- IAATO (International Association of Antarctica Tour Operators) 2013. Number of visitors (tourists, staff and crew) per site per vessel—all Antarctic sites. Accessed on the internet at http://iaato.org/tourism-statistics on 10 March 2013.
- Johnston F.M. & Pickering C.M. 2001. Alien plants in the Australian Alps. Mountain Research and Development 21, 284–291.
- Lee J.E. & Chown S.L. 2009a. Breaching the dispersal barrier to invasion: quantification and management. Ecological Applications 19, 1944–1959. Publisher Full Text
- Lee J.E. & Chown S.L. 2009b. Quantifying the propagule load associated with the construction of an Antarctic research station. Antarctic Science 21, 471–475. Publisher Full Text
- Lewis-Smith R.I. 1996. Introduced plants in Antarctica: potential impacts and conservation issues. Biological Conservation 76, 135–146. Publisher Full Text
- Lityńska-Zając M., Chwedorzewska K.J., Olech M., Korczak-Abshire M. & Augustyniuk-Kram A. 2012. Diaspores and phyto-remains accidentally transported to the Antarctic Station during three expeditions. Biodiversity and Conservation 21, 3411–3421. Publisher Full Text
- Londsale W.M. 1999. Global patterns of plant invasions and the concept of invisibility. Ecology 80, 1522–1536.
- Longeri L., Etchevers J. & Venegas J. 1979. Metodología de perfusión para estudios de nitrificación en suelo. (Perfusion method for soil nitrification studies.) Ciencia e Investigación Agraria 6, 295–299.
- Molina-Montenegro M.A., Carrasco-Urra F., Rodrigo C., Convey O., Valladares F. & Gianoli E. 2012. Occurrence of the non-native annual bluegrass on the Antarctic mainland and its negative effects on the native plants. Conservation Biology 26, 717–723. PubMed Abstract | Publisher Full Text
- Molina-Montenegro M.A., Quiróz C.L., Torres-Díaz C. & Atala C. 2011. Functional differences in response to drought in the alpine invasive Taraxacum officinale from native and introduced habitat ranges. Plant Ecology and Diversity 4, 37–44. Publisher Full Text
- Olech M. 1996. Human impact on terrestrial ecosystem in west Antarctica. Proceedings of the NIPR Symposium on Polar Biology 9, 299–306.
- Olech M. & Chwedorzewska K.J. 2011. The first appearance and establishment of an alien vascular plant in natural habitats on the forefield of a retreating glacier in Antarctica. Antarctic Science 23, 153–154. Publisher Full Text
- Pauchard A., Kueffer C., Dietz H. & MIREN Consortium. 2009. Ain’t no mountain high enough: plant invasions reaching high elevations. Frontiers in Ecology and Environment 7, 479–486. Publisher Full Text
- Resler L.M., Butler D.R. & Malanson G.P. 2005. Topographic shelter and conifer establishment and mortality in an alpine environment, Glacier National Park, Montana. Physical Geography 26, 112–125. Publisher Full Text
- Richardson D.M., Pysek P., Rejmánek M., Barbour M.G., Panetta F.D. & West C.J. 2000. Naturalization and Invasion of alien plants: concepts and definitions. Diversity and Distributions 6, 93–107. Publisher Full Text
- Robarge W.P., Edwards A. & Johnson B. 1983. Water and waste water analysis for nitrate via nitration of salicylic acid. Communication in Soil Science and Plant Analysis 14, 1207–1215. Publisher Full Text
- Robinson S.A., Wasley J. & Tobin A.K. 2003. Living on the edge—plants and global change in continental and maritime Antarctica. Global Change Biology 9, 1681–1717. Publisher Full Text
- Scott J.J. & Kirkpatrick J.B. 2005. Changes in Subantarctic Heard island vegetation at sites occupied by Poa annua, 1987–2000. Arctic, Antarctic, and Alpine Research 37, 366–371.
- Stanton M.L., Rejmánek M. & Galen C. 1994. Changes in vegetation and soil fertility along a predictable snowmelt gradient in the Mosquito Range, Colorado, USA. Arctic and Alpine Research 26, 364–374. Publisher Full Text
- Theoharides K.A. & Dukes J.S. 2007. Plant invasions across space and time: factors affecting nonindigenous species success during four stages of invasions. New Phytologist 176, 256–273. PubMed Abstract | Publisher Full Text
- Tin T., Fleming Z., Hughes K.A., Ainley D., Convey P., Moreno C., Pfeiffer S., Scott J. & Snape I. 2009. Impacts of local human activities on the Antarctic environment: a review. Antarctic Science 21, 3–33. Publisher Full Text
- Walton D.W.H. 1975. European weeds and other alien species in the Subantarctic. Weed Research 15, 271–282. Publisher Full Text
- Wasley J.S., Robinson A., Lovelock C.E. & Popp M. 2006. Climate change manipulations show Antarctic flora is more strongly affected by elevated nutrients than water. Global Change Biology 12, 1800–1812. Publisher Full Text
- Whinam J., Chilcott N. & Bergstrom D.M. 2005. Subantarctic hitchhikers: expeditioners as vectors for the introduction of alien organisms. Biological Conservation 121, 207–221. Publisher Full Text
- Zar J.H. 1999. Biostatistical analysis. 4th edn. New Jersey: Prentice-Hall.





