Dental microwear textures: reconstructing diets of fossil mammals


Published: April 1, 2016
Latest article update: Dec. 23, 2022
Abstract
Dietary information of fossil mammals can be revealed via the analysis of tooth morphology, tooth wear, tooth geochemistry, and the microscopic wear patterns on tooth surfaces resulting from food processing. Although dental microwear has long been used by anthropologists and paleontologists to clarify diets in a diversity of mammals, until recently these methods focused on the counting of wear features (e.g., pits and scratches) from two-dimensional surfaces (typically via scanning electron microscopes or low-magnification light microscopes). The analysis of dental microwear textures can instead reveal dietary information in a broad range of herbivorous, omnivorous, and carnivorous mammals by characterizing microscopic tooth surfaces in three-dimensions, without the counting of individual surface features. To date, dental microwear textures in ungulates, xenarthrans, marsupials, carnivorans, and primates (including humans and their ancestors) are correlated with known dietary behavior in extant taxa and reconstruct ancient diets in a diversity of prehistoric mammals. For example, tough versus hard object feeding can be characterized across disparate phylogenetic groups and can distinguish grazers, folivorous, and flesh consumers (tougher food consumers) from woody browsers, frugivores, and bone consumers (harder object feeders). This paper reviews how dental microwear textures can be useful to reconstructing diets in a broad array of living and extinct mammals, with commentary on areas of future research.
Keywords
Teeth, dental microwear, mammals, surface metrology, DMTA, dental microwear textures
1. Introduction
Understanding the paleobiology and ecology of ancient mammals is critical to piecing together the context of their evolutionary history, including how they responded to environmental and climatic changes over deep time. Since the extinction of non-avian dinosaurs ∼65 million years ago, mammals have evolved to fill a diversity of ecological and dietary niches on the land and in the water. Over time some herbivorous mammals became increasingly more specialized (e.g., evolving higher crowned teeth) to take advantage of abundant food resources, including the expansion of silica containing grasses at various regions across the globe during the Oligocene and Miocene (MacFadden 1992, MacFadden and Cerling 1994, Janis et al 2000, DeSantis and MacFadden 2007, Strömberg et al 2013). While tooth morphology and other craniodental features have typically been used to assess diet by comparing them to extant mammals with known dietary behavior (Mendoza et al 2002), tooth morphology is only capable of providing insight regarding the potential diet of a given species. In addition to morphological studies, alternative proxy methods are typically employed to assess the dietary ecology of fossil mammals at a given place and time, including the analysis of stable isotopes, dental mesowear, and dental microwear.
Stable isotopes from tooth enamel have the potential to reveal critical dietary information and have clarified the timing of C4 grassland expansions throughout the globe during the late Miocene and early Pliocene via carbon isotopes from fossil herbivore teeth (Cerling et al 1997). However, carbon isotopes cannot differentiate between grasses and leafy browse when both contain similar isotopic signatures (i.e., prior to the expansion of C4 grasses and/or in regions where C3 grasses or C4 shrubs are present, typically in cooler and drier regions, respectively; Ehleringer et al 1997). Dental mesowear, the macroscopic wear of tooth cusps due to attritive tooth-on-tooth wear and abrasive food-on-tooth wear (due to abrasives in or on food materials), has the potential to differentiate between grazers and browsers in living and fossil mammals (Fortelius and Solounias 2000). Specifically, tooth cusps are classified into shape (short, round, and blunt) and relief (high and low) categories or a numerical value that integrates these shape and relief classifications (typically 0–6; Mihlbachler et al 2011). Since the development of dental mesowear, the efficacy of the method has been demonstrated in extant ungulates with selenodont dentition (e.g., bison, bovids, equids, camelids, etc; Kaiser and Fortelius 2003, Kaiser and Solounias 2003, Rivals et al 2007, Blondel et al 2010, Semprebon and Rivals 2010, Mihlbachler et al 2011, Louys et al 2012, Saarinen et al 2015), extant macropods (e.g., kangaroos, wallabies, and their relatives; Butler et al 2014), and applied to a variety of extinct mammals (Croft and Weinstein 2008, Loffredo and DeSantis 2014). Specifically, teeth with blunter shapes and lower relief values are associated with abrasive food materials like grasses while teeth with sharper shapes and higher relief values are associated with browsing diets—with teeth accumulating mesowear over the lifetime of the animal. Dental mesowear is an inexpensive and fairly easy method to learn (see Loffredo and DeSantis 2014 for a discussion regarding the ability of novices to learn mesowear) and subsequently apply to assess the ecology of fossil mammals, allowing for the inclusion of hundreds to thousands of specimens in certain analyses (e.g., Mihlbachler et al 2011). However, dental mesowear assignments are fairly coarse and may not be useful in assessing intra-population differences or more nuanced dietary behavior in both living and fossil taxa (Loffredo and DeSantis 2014). Although macroscopic tooth wear proxies have been less well developed and applied in extant and extinct carnivorous mammals, macroscopic tooth wear of carnivores has similar benefits and constraints as dental mesowear—providing an inexpensive and easy method that can reveal coarse dietary information including the relative role of scavenging in the diet of a given population or species (Binder and Van Valkenburgh 2010).
Dental microwear, in contrast to the above mentioned proxy methods, provides a record of the dietary behavior of an animal shortly before its death (i.e., over the past few days to weeks; Grine 1986). Through the examination of the microscopic features on the wear facets of tooth surfaces, dietary behavior can be inferred via the examination of individual features in two dimensions (e.g., Walker et al 1978, Grine 1981, 1986, Solounias and Semprebon 2002) or the textural properties of three-dimensional surfaces (e.g., Ungar et al 2003, Scott et al 2005, 2006, Schulz et al 2010, Purnell et al 2012). Since the late 1970s, dental microwear has predominantly been used by anthropologists to assess the dietary behavior in living primates and extinct human ancestors using SEMs to record dental microwear at high magnification (∼100–500x magnification; e.g., Walker et al 1978, Grine 1981, 1986, see review by Teaford and Ungar 2000). Vertebrate paleontologists have more recently adopted a lower-magnification (∼35x) dental microwear method (Solounias and Semprebon 2002) that similarly identifies, counts, and sometimes measures individual features via a light microscope to categorize mammals as consuming grass, browse, fruit, or some mixture of these food types. For example, in herbivorous mammals, a high incidence of scratches relative to pits is interpreted as indicative of a diet of tougher food items, potentially with higher silica or grit content, in contrast to surfaces with a higher frequency of pits that instead indicate the consumption of more brittle objects including woody material, seeds, and/or fruit pits (e.g., Walker et al 1978, Grine 1981, 1986, Solounias and Semprebon 2002; also see figure 1). Although some early work has examined the dietary behavior of carnivorous mammals including living and fossil feliforms (Van Valkenburgh et al 1990, Anyonge 1996, Goillot et al 2009) and carnivorous marsupials (Robson and Young 1990), the majority of two-dimensional dental microwear research has focused on herbivorous mammals. While the efficacy of dental microwear proxy methods to infer diets in fossil mammals has largely been accepted (although not without controversy, e.g., Lucas et al 2013, Xia et al 2015), issues with observer variability (i.e., inconsistent wear feature counts between different observers) have led researchers to question the use of this tool to infer diets of mammals with more complex dietary behavior (e.g., primates) or in mammals where the size and depth of features may be decisive (Ungar et al 2003, Scott et al 2005, 2006, Mihlbachler et al 2012, DeSantis et al 2013, 2015, Donohue et al 2013).
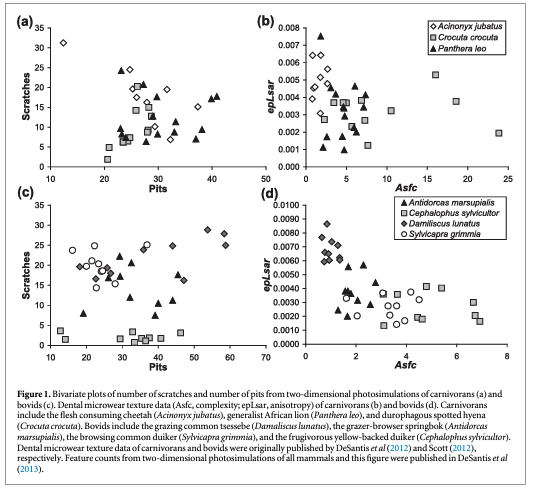
Dental microwear texture analysis (DMTA) typically uses an optical profiler to assess three-dimensional tooth surfaces via standardized surface parameters (including scale-sensitive fractal analysis (SSFA) and 3D areal surface texture standard variables; e.g., Ungar et al 2003, Scott et al 2005, 2006, Schulz et al 2010, Purnell et al 2012). It represents a significant advance over traditional SEM microwear analyses and low magnification methods, which rely on identifying microwear features such as pits and scratches from two-dimensional micrographs or from a 35x stereoscope microscope, respectively. Although 2D high-resolution SEM and 2D low magnification microwear methods have dominated in the fields of anthropology and vertebrate paleontology, respectively, these methods are less able to detect taphonomic damage or problems with molding/casting materials, are prone to observer variability issues, and may be unable to resolve dietary differences between taxa with complex diets (e.g., Mihlbachler et al 2012, DeSantis et al 2013). In contrast, DMTA analyzes tooth surfaces according to standardized parameters (SSFA parameters or 3D areal surface texture standard variables); offering a repeatable and automated method of quantifying microwear features that minimizes observer bias (Ungar et al 2003, Scott et al 2005, 2006, Purnell et al 2012). As such, it has proven to be a more effective means of identifying the diets of extant taxa, as well as assigning extinct taxa to dietary categories based on comparisons with modern taxa of known diets (e.g., DeSantis et al 2013; figure 1).
Here, this paper will review dental microwear texture methods and their use in inferring the dietary behavior of fossil mammals. This paper will discuss ways in which dental microwear textures record mammalian diets in a broad array of extant herbivorous, omnivorous, and carnivorous mammals and how these modern baselines can be used to interpret the diets of fossil mammals. Specifically, the paper will include a review of published DMTA studies on ungulates (e.g., bovids, equids, etc), canids (i.e., dogs), feliforms (i.e. cats and relatives, including hyenas), marsupials (i.e., pouched mammals including kangaroos, Tasmanian devils, and other relatives), primates (i.e., monkeys, apes, humans, and relatives), ursids (i.e., bears), and xenarthrans (i.e., armadillos, sloths, and relatives). Recent papers have discussed the benefits of analyzing dental microwear in three-dimensions as compared to traditional two-dimensional methods (e.g., Ungar et al 2003, Scott et al 2005, 2006, DeSantis et al 2013); thus, this paper will only briefly summarize the benefits of analyzing dental microwear textures in three dimensions. Further, this paper will largely focus on the use of scale-sensitive fractal properties as useful to inferring diet in extinct mammals and will only briefly mention other methods for microwear texture analysis including the use of 3D areal surface texture standard variables (ISO/FDIS 25178-2; 2012). The benefits and limitations of dental microwear texture methods will also be summarized with commentary on future research directions.
2. Dental microwear surface properties
The textural surfaces of wear facets are typically quantified and analyzed using a 3D optical profiler and either scale-sensitive fractal analysis (SSFA) or International Organization for Standardization (ISO) variables (e.g., Ungar et al 2003, Scott et al 2005, 2006, Schulz et al 2010, Purnell et al 2012, 2013, Gill et al 2014) to assess surface properties. As opposed to traditional SEM or low magnification light microscope methods where features are individually identified, counted, and sometimes measured, dental microwear texture analysis scans 3D microwear textures with a white or blue light (e.g., Ungar et al 2003, Scott et al 2005, 2006). The resulting point clouds are subsequently analyzed using ISO parameters or using SSFA software (ToothFrax and SFrax, Surfract Corporation, www.surfract.com) to characterize tooth surfaces.
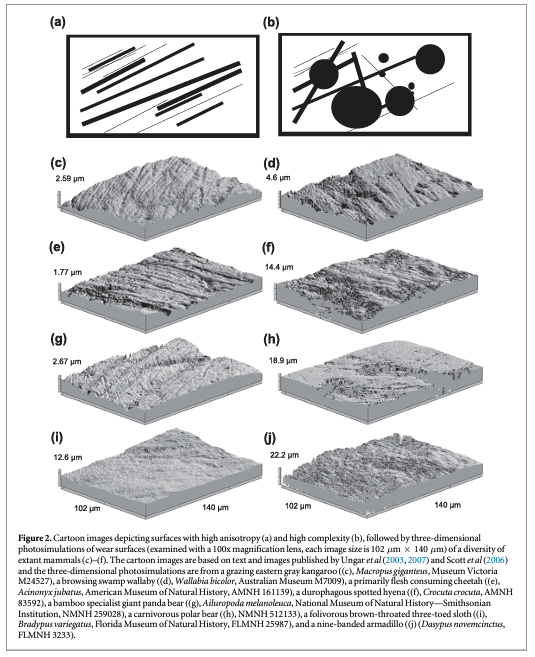
SSFA attributes include: anisotropy, complexity, scale of maximum complexity, heterogeneity, and textural fill volume. Anisotropy (exact-proportion length-scale anisotropy of relief, epLsar) represents the degree to which features share a similar orientation (e.g., lots of parallel striations yield more anisotropic surfaces; Scott et al 2005, 2006, figure 2). Specifically, anisotropy is the mean vector length resulting from quantifying the length and direction of profiles taken at 5° intervals. Grazers or flesh consumers typically have higher anisotropy due to the presence of many parallel scratches (Ungar et al 2003, Scott et al 2005, 2006, Prideaux et al 2009, Schubert et al 2010, DeSantis et al 2012, Scott 2012, Donohue et al 2013, Haupt et al 2013, DeSantis and Haupt 2014, DeSantis et al 2015). Complexity (area-scale fractal complexity, Asfc) assesses the change in surface roughness across changing scales of observation (Ungar et al 2003, Scott et al 2005, 2006), distinguishing taxa that consume brittle foods from taxa that consume softer and/or tougher ones (figure 2). Organisms that consume harder and/or more brittle food items such as woody material, seeds, fruit pits, or bone have higher complexity (Ungar et al 2003, Scott et al 2005, 2006, Prideaux et al 2009, Schubert et al 2010, DeSantis et al 2012, Scott 2012, Donohue et al 2013, Haupt et al 2013, DeSantis and Haupt 2014, DeSantis et al 2015). Scale of maximum complexity (Smc) measures the fine-scale limits of the Asfc with greater Smc surface values associated with fewer small features (Scott et al 2006). Heterogeneity (HAsfc(3×3) and HAsfc(9×9)), the degree of texture complexity variation, is measured by calculating Asfc variation among subdivided samples (a 3 × 3 and 9 × 9 grid, totaling 9–81 subsamples, respectively; Scott et al 2006). Thus, surfaces with high heterogeneity have greater disparity in complexity values between subdivided samples and the entire surface. Lastly, textural fill volume (Tfv) measures the volume filled by large (10 μm diameter) and small (2 μm diameter) square cuboids, with high Tfv values indicating potentially deeper and/or larger features (Scott et al 2006, Schubert et al 2010, DeSantis et al 2012, Scott 2012, Donohue et al 2013, DeSantis and Haupt 2014, DeSantis et al 2015).
ISO variables (ISO 25178-2 2012) include numerous attributes and between 23 and 30 variables are typically used in analyses, subsequently running multivariate analysis to separate dietary groups (Schulz et al 2010, 2013, Purnell et al 2012, 2013, Gill et al 2014). Sometimes the most powerful surface parameters are independently or collectively used to infer diets (i.e., Sal, Std, Shv, Spd, and Sq in a subset of ungulates, Schulz et al 2010; S5v, Sda, Sdq, Sdr, Sdv, Sp, Ssk, Sxp, Sz, and Vvv in rabbits with different diets, Schulz et al 2013; and Ssk, Str, Vmp, Vmc, Vvc, Vvv, Svk, Smr1, and Smr2 in extant bat species, Purnell et al 2013). Of the above mentioned examples, Ssk (skewness of height distribution of a surface, unitless) and Vvv (void volume of the core of the surface, μm3 mm−2) are both able to distinguish dietary differences in rabbits and bats. See ISO references and the work of Schulz et al (2010, 2013) and Purnell et al (2012, 2013)for a more detailed description of ISO variables. Currently, users of these methods characterize surfaces using all or a subset of ISO parameters (e.g., Schulz et al 2010, 2013, Purnell et al 2012, 2013).
SSFA attributes are well correlated with specific dietary behavior in a wide diversity of mammals (see sections 3–6), but it is less clear how certain ISO parameters correlate with specific diets. In each unique set of extant taxa different ISO parameters are useful to distinguishing between mammals with known dietary differences. Subsequently, there is little overlap or consistency in what distinguishes hard or tough object feeding across disparate mammal groups. Future work, building off Schulz et al (2010), is needed to better assess relationships between SSFA and ISO parameters. While directly comparing SSFA and ISO parameters was not a primary aim of the Schulz et al (2010) study, both sets of variables allowed discrimination between ungulates with slightly different dietary strategies. However, as the use of ISO parameters to distinguish dietary groups via the analysis of 3D surfaces is fairly recent (e.g. Schulz et al 2010, Purnell et al 2012), more work is needed to assess if ISO parameters consistently identify the consumption of food items with similar texture properties. Additionally, future work is needed to explicitly compare how SSFA and ISO parameters correlate in a variety of taxa with disparate diets. For now, because the relationship between SSFA parameters and the properties of food consumed by extant mammals is better understood (most notably, anisotropy and complexity, see sections 3–6, can distinguishing between tough and brittle food consumption, respectively) SSFA might be more informative when analyzing extinct mammals with no modern analogues or close living relatives.
3. Dental microwear of herbivorous mammals
Dental microwear textures have proven particularly valuable at differentiating between disparate diets in herbivorous mammals, most notably between grazers and browsers (e.g., Ungar et al 2007, Prideaux et al 2009, Schulz et al 2010, Scott 2012, DeSantis et al in review). Traditional dental microwear methods are often able to distinguish between mammals with broadly different diets, specifically separating taxa with increased scratch counts (resulting from the consumption of silica rich grasses) from those with increased pitting (due to browsing on woody material; e.g., Solounias and Semprebon 2002). Similarly, DMTA methods have documented increased anisotropy and decreased complexity with increased grass consumption in bovids (Ungar et al 2007, Scott 2012), ungulates (Schulz et al 2010), and macropods (Prideaux et al 2009, DeSantis et al in review, figure 2). Initial work by Ungar et al (2007) compared open-country grazers to mixed or more closed-habitat browsers, demonstrating that the open-country grazers had higher anisotropy than closed-habitat browsers, while browsers had greater complexity values than grazers. Subsequently, extinct bovids from South Africa could then be compared to modern bovids with some more closely resembling grazers, browsers, and mixed feeders (Ungar et al 2007). Scott (2012) expanded on the modern baseline of extant bovids by including 25 species ranging in dietary classifications from obligate grazers, browsers, fruigovers, and various combinations of the above mentioned groups, demonstrating a reduction in anisotropy and increased complexity with reduced toughness and increased brittleness of food items. Although the textural properties of food consumed do overlap in bovids with known differences in diet, DMTA methods can more clearly distinguish and predict known diets in extant bovids than more traditional scratch and pit counts (DeSantis et al 2013; figure 1). Further, inter-individual dietary variations in cervids (the extant roe deer, Capreolus caprelous) have also been recorded between sexes and seasons (Merceron et al 2010); thus, demonstrating the efficacy of DMTA methods in identifying subtle dietary differences in extant taxa.
Dental microwear texture patterns observed in extant bovids are also observed in extant macropods (Prideaux et al 2009, DeSantis et al in review), with the grazing eastern gray kangaroo (Macropus giganteus) demonstrating higher anisotropy and lower complexity than browsing macropods (quokkas, Setonix brachyurus; swamp wallabies, Wallabia bicolor, figure 2). Further, kangaroos with more variable diets (e.g., the western gray kangaroo, Macropus fuliginosus) are intermediate between grazers and browsers (DeSantis et al in review). While stable isotopes from mammalian tooth enamel can be used to differentiate between C4 grazers and C3 browsers when neither C3 grasses nor C4 browse are present in any abundance, much of Australia's floral landscape contains C3 grasses or C4 browse and consequently requires the use of both proxy methods to infer dietary ecology of herbivorous marsupials. Specifically, C3 grass consumption cannot be differentiated from C3 shrubs/browse consumption (typically both are present at high latitudes). Similarly, the presence of C4 shrubs (e.g., the saltbush Atriplex) in more arid regions (often found throughout Australia and in North America's southwest) can also make identifying grazers difficult by carbon isotopes alone. Using DMTA alone, one can differentiate between grass and browse consumption via anisotropy and complexity values, while the combination of both stable isotopes and DMTA can instead reveal the degree to which herbivorous mammals consumed C3 grass, C3 browse, C4 grass, and/or C4 browse (Prideaux et al 2009, DeSantis et al in review).
The integration of DMTA and isotopic methods has proven particularly valuable in revealing the dietary ecology of extinct macropods. Specifically, the extinct giant short-faced kangaroo Procoptodon goliah (a ∼ 2 m tall kangaroo that lived during the Pleistocene in Australia) was interpreted to have consumed a large proportion of C4 browse. Dental microwear texture comparisons with grazing and browsing kangaroos revealed that the giant short-faced kangaroo had significantly lower anisotropy and higher complexity than grazing and browsing macropods (Prideaux et al 2009). Reliance on C4 browse may have made Procoptodon goliah more vulnerable to extinction due to its need to supplement its water intake with water from lakes and/or ponds (due to the salty nature of saltbush), either due to increased vulnerability to hunting while at watering holes (Prideaux et al 2009) or a reduction of watering holes due to increased aridification.
Currently, DMTA baselines are being expanded to include a broad array of artiodactyls and perissodactyls (DeSantis and Schubert 2015, Jones and DeSantis 2015). With the improved ability of optical 3D profilers to examine surfaces at 150x magnification, as opposed to 100x magnification, researchers are working to expand DMTA methods to include small mammals, including a diversity of rodents and lagomorphs. With the ability to infer diet in small mammals, floral landscapes can be inferred at large scales via megafauna (animals >44 kg) and smaller patches via smaller microfauna. Further, the effects of climate change on large and small mammals can be assessed and compared. Future studies on the DMTA of small mammals may help reveal a more complete picture of floral landscapes during periods of pronounced climate change or evolutionary innovations, including a better context of human evolution and the global extinction of Pleistocene megafauna.
4. Dental microwear of carnivorous mammals
Inferring dietary behavior of carnivores, including high degrees of bone processing, is possible using DMTA. Schubert et al (2010) demonstrated the ability to use DMTA of specialized slicing teeth (i.e., carnassials) to assess relative bone consumption in extant African feliforms, including demonstrating higher anisotropy and lower complexity and textural fill volume in flesh consuming cheetahs (Acinonyx jubatus) as compared to more generalized African lions (Panthera leo), and spotted hyenas (Crocuta crocuta) which are known to scavenge and process bone (figures 1 and 2). These results are consistent with differences in bone consumption rates among the three species; thus, the application of microwear analyses to carnivores can be used to interpret competition and niche position within a guild of fossil carnivores across space and through time. The extant baseline of feliforms has since been expanded (DeSantis et al 2012, DeSantis and Haupt 2014), continuing to demonstrate the efficacy of DMTA at inferring diets in extant carnivorans. Further, direct comparisons of DMTA with traditional 2D methods have revealed the importance of analyzing dental microwear surfaces of carnivorous mammals in three-dimensions (DeSantis et al 2013, figure 1). Specifically, individual feature counts of pits tend to increase with more bone processing; however, with significant bone processing (i.e., in taxa known to scavenge), pits often become larger but subsequently decrease in pit density due to pit size increases (DeSantis et al 2013). Thus, spotted hyenas have fewer pits than both cheetahs and African lions despite increased durophagy (i.e., bone processing; DeSantis et al 2013, figure 1). DMTA instead distinguishes feliforms with different degrees of bone processing (Schubert et al 2010, DeSantis et al 2012, 2015, DeSantis and Haupt 2014) and is useful for clarifying the paleobiology of extinct carnivores. Most notably, DMTA of the American lion (Panthera atrox) and saber-toothed cat (Smilodon fatalis) from the La Brea Tar Pits in southern California demonstrate that extinct cats were not desperately consuming carcasses shortly before their extinction (DeSantis et al 2012). Instead, the absence of dental microwear with high 'hyena-like' complexity values questions the idea that tough-times led to their extinction (as initially proposed by Van Valkenburgh and Hertel 1993). Dental microwear of cougars (Puma concolor) suggest that more opportunistic diets, much like cougars exemplify today and was the case in the past, may have been key to their survival through the late Pleistocene megafaunal extinction (DeSantis and Haupt 2014).
Similar to cats and their relatives, DMTA can be used to clarify the dietary ecology of extinct canids. However, recent work has demonstrated the need to carefully consider the function of different teeth when assessing dietary behavior via DMTA. For example, the lower second molar (m2) which is primarily used for crushing and grinding is useful for capturing dietary behavior in contrast to the carnassial facet (Ungar et al 2010). Similar to dogs, tooth function plays an important role in determining if DMTA can record dietary behavior in bears and their relatives. Ursids are a unique group of carnivorans in having evolved to fill a variety of dietary niches ranging from predominantly herbivorous giant panda bears (Ailuropoda melanoleuca) to herbivorous/omnivorous sun bears (Ursus malayanus) and spectacle bears (Tremarctos ornatus) and primarily omnivorous/carnivorous black bears (Ursus americanus), brown bears (Ursus arctos), and polar bears (Ursus maritimus; Donohue et al 2013), in a fairly short period of time (∼5.3 million years ago there was an explosive radiation of extinct and extant bears; Krause et al 2008). However, like dogs, the first and second lower molars serve different functions, with the first molar carnassial shearing facet often functioning as a tool in giant panda bears while used to slice meat in polar bears (Donohue et al 2013). By examining the dental microwear textures of the buccal facet of the lower first molar (m1) protoconid and the mesial facet of the m2 hypoconulid, Donohue et al (2013) confirmed that dental microwear textures are recorded differently in different teeth of the same individual, as consistent with tooth function. Unlike the dental microwear textures on m2s, which are able to distinguish between bamboo specialists, more herbivorous/frugivorous bears, and more omnivorous/carnivorous bears (figure 2), the m1 was unable to differentiate bears with disparate diets. Thus, to evaluate diets in extinct bears the examination of m2 grinding facets (or the occluding facets on upper third molars) is necessary. Dental microwear textures of the m2s of the giant short-faced bear Arctodus simus suggest that this giant bear (∼700–800 kg in size; Christiansen 1999) may have consumed more plant material than previously thought and is in agreement with morphological data (Figueirido et al 2010), with lower variance of complexity than the more omnivorous/carnivorous black bears and polar bears and instead most similar to its closest living relative the Tremarctine spectacled bear (Donohue et al 2013). While further work is needed to evaluate the degree to which Arctodus simus consumed meat (ideally via additional nitrogen isotope analyses across their geographic range), DMTA data clearly demonstrate that it was not a hyper-scavenger and did not specialize on hard/brittle objects like bones (in contrast to the hyper-scavenger hypothesis proposed by Matheus 1995). Currently, the baseline of extant bears is being expanded to include additional taxa, individuals from a broad geographic range, and specimens collected during different seasons (e.g., Smith et al 2015a).
In addition to examining true carnivorans, DMTA can also be useful to clarifying dietary behavior in non-carnivorans including carnivorous marsupials. For example, Tasmanian devils (Sarcophilus harrisii) are observed to fully consume carcasses and have dental microwear textures consistent with dietary behavior: low anisotropy, high complexity, and high textural fill volume (Jiang and DeSantis 2014). Further, dental microwear textures do not vary between teeth with similar tooth functions, specifically the 'carnassial-like' second, third, and fourth molars, despite differential placement in the jaw. Although Jiang and DeSantis (2014) hypothesized that Tasmanian devils may have more complex dental microwear surfaces further back in the jaw where bite force estimates and stresses are highest (Attard et al 2011), second through fourth lower molars are indistinguishable from one another. By combining morphological analyses (including finite element analysis) with dental microwear texture analysis, dietary behavior in carnivorous marsupials can be resolved through time and across space. Current work is aimed at assessing if and how the dietary behavior of Tasmanian devils and thylacines (both members of the order Dasyuromorphia) changed when restricted to Tasmania and/or with the recent arrival of Europeans in the 17th century (DeSantis 2014).
5. Dental microwear of mammals with complex diets
Constant questions surrounding the complex dietary ecology of primates, including humans and their ancestors, abounded within anthropology. Since primates occupy a wide variety of dietary niches (many defined as folivorous, frugivorous, insectivorous, and omnivorous, including preferred and fallback resources), it was imperative to distinguish not only general dietary categories, but also subtle differences due to seasonality or individual inclination. While traditional 2D approaches have typically been successful at differentiating between general categories, 3D methods have provided evidence for fine-scale variation and are critical to advancing the study of our closest relatives. For example, early work by Scott et al (2005) demonstrated that the more folivorous mantled howler monkey (Alouatta palliata) could be distinguished from the tufted capuchin monkey (Cebus apella), a consumer of fruits and brittle seeds, in having higher anisotropy and variance of anisotropy and lower complexity and variance of complexity. These clear differences between extant primates allowed for comparisons of early hominins including Australopithecus africanus, Paranthropus boisei, and Paranthropus robustus. Morphological analyses of the colloquially named 'nutcracker man' (i.e., Paranthropus boisei) suggested that these hominins with pronounced sagittal crests (with large attachments for temporalis muscles, associated with higher bite forces) and large flat teeth were well adapted for consuming hard objects—potentially including underground tubers (e.g., Wood and Constantino 2007, Smith et al 2015b). However, DMTA analysis of P. boisei instead demonstrates that it ate softer and/or tougher foods than A. africanus (Ungar et al 2008). Further, the hominins Australopithecus africanus and Paranthropus robustus did not consume food items with vastly different textural attributes, as was previously thought to be the case (Scott et al 2005). Dental microwear texture data of hominids collectively questions the idea that the large teeth of Paranthropus species (specifically, Paranthropus boisei; Scott et al 2005, Ungar et al 2012) were used for hard object consumption and instead further support the idea that their craniodental morphology was an adaptation for prolonged chewing of tougher food items (similar to suggestions made by Cerling et al 2011 as evinced by geochemical data) or the rare consumption of hard objects (an example of Liem's paradox where their 'highly derived morphology need not reflect a specialized diet'; Ungar et al 2008).
The dental microwear baseline of primates has exploded since the development of DMTA and now includes numerous monkey, apes, and even prehistoric and modern humans (also see review by Scott et al 2012). Dietary analysis of dental microwear textures of primates is typically restricted to the analysis of Phase II facets (i.e., 9, x, and 10n; Kay 1977) on molar teeth, as intraspecific and intra-individual differences in microwear can be extreme (Gordon 1984). However, teeth other than molars can also prove informative. For example, work by Krueger and Ungar have examined how modern humans and hominins (including Neanderthals) may have used their incisors as potential tools (Krueger and Ungar 2012, Krueger 2015). Dental microwear textures from incisors were able to differentiate dietary and behavioral practices in modern human populations (Krueger 2015). When Neanderthals were compared to bioarchaeological/ethnographic groups with different diets, dental microwear textures of anterior teeth suggest that the Krapina Neanderthal only periodically used their incisors for non-diet related behaviors (Kruger and Ungar 2012). El-Zaatari (2010) also demonstrated how DMTA captures differences in diets and food preparation techniques between historic and modern populations of humans. For example, native Tigara samples have higher complexity values than the Fuegians and Chumash samples, despite all mainly hunting and gathering (including consuming marine mammals and fish). Unlike Fuegians who primarily eat fresh marine protein shortly after the food is acquired, Tigara people often preserve marine mammals in abrasive sand; thus, the abrasives on food results in different dental microwear textures in human populations (El-Zaatari 2010). Similarly, early Peruvians from Huaca Prieta who ate marine food materials and likely cooked terrestrial plant material also show dental microwear textures more similar to Fuegians, as compared to other human populations (DeSantis et al in press).
6. Dental microwear of dentin
Prior to the late Pleistocene megafaunal extinction, giant ground sloths and armadillo-like animals used to roam much of North and South America. These mammals, members of the superorder Xenarthra, often attained sizes of >1000 kg with some nearly 4000 kg in size (Pant et al 2014) and were a dominant part of many ecosystems. Yet, our understanding of their biology and subsequent impacts on ecosystems is often limited to inferences based on morphological studies—in contrast to other co-occurring megafauna where the above mentioned proxies are often employed (see section 1). Clarifying the paleobiology and paleoecology of extinct giant ground sloths and giant armadillos is particularly challenging. In addition to the lack of appropriate modern analogues (i.e., all extant xenarthrans have potentially very different ecologies than extinct forms and are dramatically smaller in body size), xenarthrans do not have tooth enamel. The lack of enamel limits the use of paleoecological proxies mentioned above as dentin is softer and more organic rich, posing problems to both dental microwear and isotopic analyses. Specifically, the isotopic composition of tooth enamel may be more diagenetically altered due to the higher organic content and greater pore space in dentin tissues (Wang and Cerling 1994); although, work by MacFadden et al (2010) has shown that dentin is not always more altered than enamel and may vary depending on the type of xenarthran sampled and between fossil localities (which may vary in age, local soil/mineral composition, and hydrologic properties that could all affect diagenesis). Similarly, the microscopic wear patterns on teeth may be recorded differently from enamel and/or may be more prone to taphonomic alteration.
Work by Haupt et al (2013) tested the idea that dentin and enamel record dental microwear textures differently by examining carnassial teeth in cougars that are subjected to similar shearing forces and food items. Carnassial teeth of carnivorans were preferred over ungulate teeth, as dentin and enamel in ungulate teeth are typically not exposed along the same geometric plane and are therefore not exposed to identical chewing forces. Differences were observed between enamel and dentin on identical teeth, in cougars (Haupt et al 2013); thus, dentin and enamel may not be directly comparable. However, tooth dentin microwear of extant folivorous sloths (Hoffmann's two-toed sloth Choloepus hoffmanni and the three-toed brown-throated sloth Bradypus variegatus) could be distinguished from the more insectivorous and ground dwelling nine-banded armadillo in having lower complexity (Dasypus novemcinctus; Haupt et al 2013). While work on extant xenarthrans appears promising, future work is needed to resolve if dentin is able to capture and preserve dental microwear over deep time. If dental microwear textures do prove useful in revealing diets in fossil taxa, understandings of the paleoecology of xenarthrans will be dramatically improved.
7. Discussion and future research
Regardless of trophic group or phylogenetic history, dental microwear textures are capable of revealing the dietary ecology of extant mammals with known diets. Specifically, the consumption of harder/brittle food items can be distinguished from tougher food consumers with the textural properties of complexity and anisotropy, respectively. As many fossil mammals lack modern analogues necessary to test the how DMTA or ISO properties reflect diet, the ability to infer the textural properties of food consumed in a broad array of extant and extinct mammals makes the use of DMTA attributes invaluable. Further, the fact that all extant mammals examined record the textural properties of food consumed consistent with their diet, despite varying levels of grit on the landscape, suggests that the consumption of dietary food items is driving the microwear signal recorded on teeth. Further work is needed to resolve the potential role of grit in dental microwear textures. Most work, to date, has focused on the role of grit in dental microwear as captured in two-dimensions (e.g., Kay and Covert 1983, Ungar et al 1995, Lucas et al 2013, Hoffmann et al 2015). However, recent studies have clearly demonstrated that dental microwear can be formed by food items (Xia et al 2015), in contrast to prior work which has called this into question (Lucas et al 2013).
Currently, much research is aimed at improving our understanding of how mammals have responded to long-term climate change, including responses to glacial cooling and interglacial warming during the Pleistocene, rapid warming associated with the Paleocene-Eocene thermal maximum (∼55 million years ago), and long-term aridification on continents like Australia (since the Miocene). With methodological advances, including the ability to scan and process more individual surfaces in less time, the reduced costs of DMTA can help facilitate the examination of more specimens. Assessing how mammals have altered their diet during periods of pronounced climate change may be able to answer questions regarding current biotic responses to climate change.
It may also be valuable to take advantage of the short-time window captured by dental microwear analyses. As dental microwear from the past few days to weeks of an animal's life are captured shortly before its death, future studies can exploit these data to assess how extant mammals may be responding to pronounced changes in climate (e.g., extreme drought events) or how diets of extant mammals vary seasonally (similar to Merceron et al 2010). Current work of some researchers is aimed at comparing how the textural properties of food consumed by kangaroos changed during pronounced drought events—by comparing kangaroos culled under healthy 'normal' conditions as compared to those that died during extreme droughts (DeSantis 2015). In elusive mammals that are particular challenging to study, like solitary tapirs or bears, DMTA can help clarify if and how their diets vary seasonally (similar to Merceron et al 2010). Additionally, with current global warming impacting mammals across the globe, DMTA may help reveal how historic changes in climate have impacted mammalian diets.
As mentioned in section 4, there is a critical need to consider how tooth function may influence dental microwear and if dental microwear from different teeth or wear facets is capturing observed dietary differences in extant taxa. While carnivorans differentiate molar teeth for different functions, with dogs and bears having lower second molars well adapted for crushing and grinding, other carnivorous mammals have similar microwear on teeth with similar functions—as is the case in marsupial carnivores like the Tasmanian devil (Jiang and DeSantis 2014). Although much work is needed to assess if bovids, kangaroos, and numerous other groups have different microwear on more anterior or posterior molars (and facets), the similarity of molar teeth within a given individual (e.g., m2 versus m3) suggests that tooth function is likely similar between these teeth (in a given taxon) and dental microwear textures may also be similar. Thus, microwear from an individual wallaby m2 and m3 may be similar to one another. Some macropods exhibit molar progression where teeth erupt and subsequently move forward in the jaw as additional teeth erupt (Sanson and Miller 1979). Although future work is needed to assess if and how dental microwear varies in early versus later erupting teeth, the exact tooth number may be less of a factor than its position in the jaw at a given point in time. While it is critical to understand how tooth position and facet may influence dental microwear, it is also important to recognize limitations of the fossil record and the consequences of restricting DMTA analysis to a specific tooth and facet—especially if no functional differences between teeth are known in the study organism. Further, relationships between macroscopic wear of teeth and dental microwear may reveal if and how mammals alter their diet with age. Increased age and tooth wear may require certain mammals to alter their diet and eat non-preferred food items; thus, clearly quantifying macroscopic wear in teeth also examined for DMTA may help clarify the ecology of living and fossil mammals. That being said, the examination of certain teeth and wear facets may not be possible if teeth have too little or too much wear, and these constraints must also be considered.
Despite methodological advances in the analysis of dental microwear textures, most notably the ability to move away from the identification, counting, and measurement of individual features, some limitations have become apparent. Specifically, confocal microscopes and optical profiles can vary in the size of scans, resolution of measurements, and ability to quantify, edit, and analyze surfaces. Further, identical machines quantify surfaces differently if light options (blue versus white), thresholds, scan size, and other algorithms are selected/applied differently (Ungar et al 2014, Arman et al 2015). While microscope variability concerns may seem comparable to observer variability issues of 2D dental microwear methods, most of these issues can be overcome by sharing 'recipes' (a record of all parameters selected) and loading of like 'recipes' between individuals and labs with similar profilers. However, when confocal microscopes or profilers are dramatically different—further work is needed to assess if and how microscope variability is significant between research labs. Additionally, more work is needed to compare SSFA (Asfc, epLsar, Smc, Tfv, and HAsfc) to ISO variables (ISO 25178-2) to better understand if certain ISO properties are consistently able to differentiate dietary information in a diverse suite of mammals, and their relationship to DMTA attributes.
8. Conclusions
The analysis of dental microwear textures in three-dimensions has allowed for an improved understanding of the ecology of ancient mammals, including humans and our ancestors. Since the development of DMTA and other textural analyses using ISO parameters, much of the current research has focused on expanding necessary extant baselines to better interpret the dietary ecology of herbivores, carnivores, and a variety of mammals with more complex diets. Additionally, the examination of fossil and historic specimens have altered ideas regarding the cause of megafaunal extinctions, the dietary behavior of early hominids, and have clarified the context of mammalian evolution with a more complete picture of mammalian dietary ecology. It is reassuring that in all published cases, the dental microwear textures of extant mammals match with observed dietary behavior in primates, xenarthrans, ungulates, marsupials, and carnivorans, with higher complexity and anisotropy tracking the consumption of harder/brittle objects and tougher food items, respectively. The ability for dental microwear textures to reveal the dietary behavior and ecology of a diversity of prehistoric, historic, and extant mammals (including humans) is promising and will continue to benefit from recent technological advances in optical profilers and the field of surface metrology.
Acknowledgments
Much thanks is due to P Ungar and colleagues for the development of DMTA and subsequent sharing of their knowledge and lab facilities with interested researchers, including myself. The work here reviewed would not have been possible without access to numerous publically accessible collections throughout the globe. I personally would like to thank curators and collections managers from the American Museum of Natural History, Australian Museum, East Tennessee Museum of Natural History and Gray Fossil Site, Field Museum of Natural History, Florida Museum of Natural History, Iziko South African Museum, Melbourne Museum, National Museum of Natural History—Smithsonian Institution, Natural History Museum of Los Angeles County (including the Page Museum), Queen Victoria Museum and Art Gallery, Queensland Museum, Santa Barbara Museum of Natural History, South Australian Museum, Tasmanian Museum and Art Gallery, Texas Memorial Museum, University of California Museum of Paleontology, Western Australian Museum, and Yale Peabody Museum. I am also grateful to the thorough reviews provided by anonymous reviewers. This work was partially funded by Vanderbilt University and the National Science Foundation via EAR 1053839 and 1455198.
References
- Anyonge W 1996 Microwear on canines and killing behavior in large carnivores: saber function in Smilodon fatalis J. Mammal. 77 1059–67
- Arman S, Prideaux G, Ungar P, Brown C, DeSantis L and Schmidt C 2015 Intra- and Inter- microscope differences in dental microwear texture analysis J. Vert. Paleontol. 2015 81 Program and Abstracts
- Attard M R G, Chamoli U, Ferrara T L, Rogers T L and Wroe S 2011 Skull mechanics and implications for feeding behaviour in a large marsupial carnivore guild: the thylacine, Tasmanian devil and spotted tailed quoll J. Zool. 285 292–300
- Binder W J and Van Valkenburgh B 2010 A comparison of tooth wear and breakage in Rancho La Brea sabertooth cats and dire wolves across time J. Vert. Paleontol. 30 255–61
- Blondel C, Merceron G, Andossa L, Taisso M H, Vignaud P and Brunet M 2010 Dental mesowear analysis of the late Miocene Bovidae from Toros-Menalla (Chad) and early hominid habitats in Central Africa Palaeogeogr. Palaeoclimtol. Palaeoecol. 292 184–91
- Butler K, Louys J and Travouillon K 2014 Extending dental mesowear analyses to Australian marsupials, with applications to six Plio-Pleistocene kangaroos from southeast Queensland Palaeogeogr. Palaeoclimtol. Palaeoecol. 408 11–25
- Cerling T E, Harris J M, MacFadden B J, Leakey M G, Quade J, Eisenmann V and Ehleringer J R 1997 Global vegetation change through the Miocene/Pliocene boundary Nature 389 153–8
- Cerling T E, Mbua E, Kirera F M, Manthi F K, Grine F E, Leakey M G, Sponheimer M and Uno K T 2011 Diet of Paranthropus boisei in the early Pleistocene of East Africa Proc. Natl Acad. Sci. USA 108 9337–41
- Christiansen P 1999 What size were Arctodus simus and Ursus spelaeus (Carnivora: Ursidae)? Ann. Zool. Fennici. 36 93–102
- Croft D A and Weinstein D 2008 The first application of the mesowear method to endemic South American ungulates (Notoungulata) Palaeogeogr. Palaeoclimtol. Palaeoecol. 269 103–14
- DeSantis L 2014 Dramatic dietary modifications of carnivorous marsupials in Australia as revealed by dental microwear texture analysis: potential consequences of increased competition with novel predators during the Holocene J. Vert. Paleontol. 2014 119 Program and Abstracts
- DeSantis L 2015 Mammalian responses to climate change: lessons learned from both 'deep-time' experiments and modern ecological studies J. Vert. Paleontol. 2015 116 Program and Abstracts
- DeSantis L R G, Field J H and Wroe S Dietary responses of Sahul (Pleistocene Australia-New Guinea) megafauna to climate and environmental change Paleobio. submitted
- DeSantis L R G, Dillehay T, Feranec R and Goodbred S Dietary ecology, stable isotope, and dental microwear texture analysis Where the Land Meets the Sea: 4,000 years of Human History in the Andes (Cambridge: Cambridge University Press) ed T Dillehay (in press)
- DeSantis L R G and Haupt R J 2014 Cougars' key to survival through the late Pleistocene extinction: insights from dental microwear texture analysis Biol. Lett. 10 20140203
- DeSantis L R G and MacFadden B J 2007 Identifying forested environments in Deep Time using fossil tapirs: evidence from evolutionary morphology and stable isotopes Cour. Forsch. -Inst. Senkenberg 258 147–57
- DeSantis L R G and Schubert B W 2015 Tales from tapir teeth: dietary ecology of extant and extinct tapirs as infered from dental microwear texture analysis Southeastern Vertebrate Paleontology Meeting Program and Abstracts Book 2015
- DeSantis L R G, Schubert B W, Scott J R and Ungar P S 2012 Implications of diet for the extinction of saber-toothed cats and American lions PLoS One 7 e52453
- DeSantis L R G, Schubert B W, Schmitt-Linville E, Ungar P, Donohue S and Haupt R 2015 Dental microwear textures of carnivorans from the La Brea Tar Pits, California and potential extinction implications (Science Series, Natural History Museum of Los Angeles County) 42 37–52
- DeSantis L R G, Scott J R, Schubert B W, Donohue S L, McCray B M, Van Stolk C A, Wilburn A A, Greshko M A and O'Hara M C 2013 Direct comparisons of 2D and 3D dental microwear proxies in extant herbivorous and carnivorous mammals PLoS One 8 e71428
- Donohue S L, DeSantis L R G, Schubert B W and Ungar P S 2013 Was the giant short-faced bear a hyper-scavenger? A new approach to the dietary study of ursids using dental microwear textures PLoS One 8 e77531
- Ehleringer J R, Cerling T E and Helliker B R 1997 C4 photosynthesis, atmospheric CO2, and climate Oecologia 112 285–99
- El-Zaatari S 2010 Occlusal microwear texture analysis and the diets of historical/prehistoric hunter-gatherers Int. J. Osteoarchaeol. 20 67–87
- Figueirido B, Pérez-Claros J A, Torregrosa V, Martín-Serra A and Palmqvist P 2010 Demythologizing Arctodus simus, the 'short-faced' long-legged and predaceous bear that never was J. Vert. Paleontol. 30 262–75
- Fortelius M and Solounias N 2000 Functional characterization of ungulate molars using abrasion attrition wear gradient Am. Mus. Novitat. 3301 1–36
- Gill P G, Purnell M A, Crumpton N, Brown K R, Gostling N J, Stampanoni M and Rayfield E J 2014 Dietary specializations and diversity in feeding ecology of the earliest stem mammals Nature 512 303–5
- Goillot C, Blondel C and Peigné S 2009 Relationships between dental microwear and diet in Carnivora (Mammalia)—implications for the reconstruction of the diet of extinct taxa Palaeogeog. Palaeoclimatol. Palaeoecol. 271 13–23
- Gordon K D 1984 Hominoid dental microwear: complications in the use of microwear analysis to detect diet J. Dent. Res. 63 1043–6
- Grine F E 1981 Trophic differences between gracile and robust australopithecines-a scanning electron-microscope analysis of occlusal events S. Afr. J. Sci. 77 203–30
- Grine F E 1986 Dental evidence for dietary differences in Australopithecus and Paranthropus: a quantitative analysis of permanent molar microwear J. Hum. Evol. 15 783–822
- Haupt R J, DeSantis L R G, Green J L and Ungar P S 2013 Dental microwear texture as a proxy for diet in xenarthrans J. Mammal. 94 856–66
- Hoffman J M, Fraser D and Clementz M 2015 Controlled feeding trials with ungulates: a new application of in vivo dental molding to assess the abrasive factors of microwear J. Exp. Biol. 218 1538–47
- ISO 25178-2 2012 Geometrical product specifications (GPS)—Surface Texture: Areal—: 2. Terms, Definitions and Surface Texture Parameters (International Organization for Standardization)
- Janis C M, Damuth J and Theodor J M 2000 Miocene ungulates and terrestrial primary productivity: where have all the browsers gone? Proc. Natl Acad. Sci. USA 97 7899–904
- Jiang T and DeSantis L R G 2014 Dental microwear texture analysis of the tasmanian devil: assessing variability among teeth Young Sci. 4 30–2
- Jones B and DeSantis L 2015 Dietary ecology of herbivorous megafauna from the La Brea tar pits in southern California: evidence of changing dietary behaviour coincident with climate change J. Vert. Paleontol. 2015 244 Program and Abstracts
- Kaiser T M and Fortelius M 2003 Differential mesowear in occluding upper and lower molars: opening mesowear analysis for lower molars and premolars in hypsodont horses J. Morphol. 258 67–83
- Kaiser T M and Solounias N 2003 Extending the tooth mesowear method to extinct and extant equids Geodiversitas 25 321–45
- Kay R F 1977 The evolution of molar occlusion in Cercopithecidae and early catarrhines Am. J. Phys. Anthropol. 46 327–52
- Kay R F and Covert H H 1983 True grit: a microwear experiment Am. J. Phys. Anthropol. 46 327–52
- Krause J et al. 2008 Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary BMC Evol. Biol. 8 220
- Krueger K L 2015 Reconstructing diet and behavior in bioarchaeological groups using incisor microwear texture analysis J. Archaeol. Sci. Rep. 1 29–37
- Krueger K L and Ungar P S 2012 Anterior dental microwear texture analysis of the Krapina Neandertals Cent. Eur. J. Geosci. 4 651–62
- Loffredo L F and DeSantis L R 2014 Cautionary lessons from assessing dental mesowear observer variability and integrating paleoecological proxies of an extreme generalist Cormohipparion emsliei Palaeogeogr. Palaeoclimtol. Palaeoecol. 395 42–52
- Louys J, Ditchfield P, Meloro C, Elton S and Bishop L C 2012 Stable isotopes provide independent support for the use of mesowear variables for inferring diets in African antelopes Proc. R. Soc. B 279 4441–6
- Lucas P W, Omar R, Al-Fadhalah K, Almusallam A S, Henry A G, Michael S, Thai L A, Watzke J, Strait D S and Atkins A G 2013 Mechanisms and causes of wear in tooth enamel: implications for hominin diets J. R. Soc. Interface 10 20120923
- MacFadden B J 1992 Fossil Horses—Systematics, Paleobiology, and the Evolution of the Family Equidae (Cambridge: Cambridge University Press)
- MacFadden B J and Cerling T E 1994 Fossil horses, carbon isotopes and global change Trends Ecol. Evol. 9 481–6
- MacFadden B J, DeSantis L R G, Hochstein J L and Kamenov G D 2010 Physical properties, geochemistry, and diagenesis of xenarthran teeth: prospects for interpreting the paleoecology of extinct species Palaeogeogr. Palaeoclimtol. Palaeoecol. 291 180–9
- Matheus P E 1995 Diet and co-ecology of Pleistocene short-faced bears and brown bears in eastern Beringia Quat. Res. 44 447–53
- Mendoza M, Janis C M and Palmqvist P 2002 Characterizing complex craniodental patterns related to feeding behaviour in ungulates: a multivariate approach J. Zool. 258 223–46
- Merceron G, Escarguel G, Angibault J-M and Verheyden-Tixier H 2010 Can dental microwear textures record inter-individual dietary variations? PLoS One 5 e9542
- Mihlbachler M C, Beatty B L, Caldera-Siu A, Chan D and Lee R 2012 Error rates and observer bias in dental microwear analysis using light microscopy Paleontol. Electronica 15 12A
- Mihlbachler M C, Rivals F, Solounias N and Semprebon G M 2011 Dietary change and evolution of horses in North America Science 331 1178–81
- Pant S R, Goswami A and Finarelli J A 2014 Complex body size trends in the evolution of sloths (Xenarthra: Pilosa) BMC Evol. Biol. 14 184
- Prideaux G J, Ayliffe L K, DeSantis L R G, Schubert B W, Murray P F, Gagan M K and Cerling T E 2009 Extinction implications of a chenopod browse diet for a giant Pleistocene kangaroo Proc. Natl Acad. Sci. USA 106 11646–50
- Purnell M, Seehausen O and Galis F 2012 Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes J. R. Soc. Interface 9 2225–33
- Purnell M A, Crumpton N, Gill P, Jones G and Rayfield E 2013 Within-guild dietary discrimination from 3D textural analysis of tooth microwear in insectivorous mammals J. Zool. 291 249–57
- Rivals F, Mihlbachler M C and Solounias N 2007 Effect of ontogenetic-age distribution in fossil and modern samples on the interpretation of ungulate paleodiets using the mesowear method J. Vert. Paleontol. 27 763–7
- Robson S K and Young W G 1990 A comparison of tooth microwear between an extinct marsupial predator, the Tasmanian tiger Thylacinus cynocephalus (Thylacinidae) and an extant scavenger, the Tasmanian devil Sarcophilus harrisii (Dasyuridae, Marsupialia) Aust. J. Zool. 37 575–89
- Saarinen J et al. 2015 A new tooth wear-based dietary analysis method for Proboscidea (Mammalia) J. Vert. Paleo. 35 e918546
- Sanson G D and Miller W A 1979 Mechanism of molar progression in macropods Anat. Rec. 193 674
- Schubert B W, Ungar P S and DeSantis L R G 2010 Carnassial microwear and dietary behavior in large carnivorans J. Zool. 280 257–63
- Schulz E, Calandra I and Kaiser T M 2010 Applying tribology to teeth of hoofed mammals Scanning 32 162–82
- Schulz E, Piotrowski V, Clauss M, Mau M, Merceron G and Kaiser T M 2013 Dietary abrasiveness is associated with variability of microwear and dental surface texture in rabbits PLoS One 8 e56167
- Scott J R 2012 Dental microwear texture analysis of extant African Bovidae Mammalia 76 157–74
- Scott R S, Ungar P S, Bergstrom T S, Brown C A, Grine F E, Teaford M F and Walker A 2005 Dental microwear texture analysis shows within-species diet variability in fossil hominins Nature 436 693–5
- Scott R S, Ungar P S, Bergstrom T S, Brown C A, Childs B E, Teaford M F and Walker A 2006 Dental microwear texture analysis: technical considerations J. Hum. Evol. 51 339–49
- Scott R S, Teaford M F and Ungar P S 2012 Dental microwear texture and anthropoid diets Am. J. Phys. Anthropol. 147 551–79
- Semprebon G M and Rivals F 2010 Trends in the paleodietary habits of fossil camels from the Tertiary and Quaternary of North America Palaeogeogr. Palaeoclimtol. Palaeoecol. 295 131–45
- Solounias N and Semprebon G 2002 Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids Am. Mus. Novit. 3366 1–49
- Smith G, Graham R, DeSantis L and Donohue S 2015a Bergmann's response and dietary variability in North American black bears (Ursus americanus) J. Vert. Paleontol. 119 SVP Program and Abstracts Book November 2014
- Smith A L et al. 2015b The feeding biomechanics and dietary ecology of Paranthropus boisei Anat. Rec. 298 145–67
- Strömberg C A, Dunn R E, Madden R H, Kohn M J and Carlini A A 2013 Decoupling the spread of grasslands from the evolution of grazer-type herbivores in South America Nat. Commun. 4 1478
- Teaford M F and Ungar P S 2000 Diet and the evolution of the earliest human ancestors Proc. Natl Acad. Sci. USA 97 13506–11
- Ungar P, Ragni A and DeSantis L 2014 Comparability of dental microwear texture data between studies J. Vert. Paleontol. 2014 244 Program and Abstracts
- Ungar P S, Brown C A, Bergstrom T S and Walker A 2003 Quantification of dental microwear by tandem scanning confocal microscopy and scale-sensitive fractal analyses Scanning 25 185–93
- Ungar P S, Grine F E and Teaford M F 2008 Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei PLoS One 3 e2044
- Ungar P S, Krueger K L, Blumenschine R J, Njau J and Scott R S 2012 Dental microwear texture analysis of hominins recovered by the Olduvai Landscape Paleoanthropology Project, 1995–2007 J. Hum. Evol. 63 429–37
- Ungar P S, Merceron G and Scott R S 2007 Dental microwear texture analysis of Varswater bovids and early Pliocene paleoenvironments of Langebaanweg, Western Cape Province, South Africa J. Mamm. Evol. 14 163–81
- Ungar P S, Scott J R, Schubert B W and Stydner D D 2010 Carnivoran dental microwear textures: comparability of carnassial facets and functional differentiation of postcanine teeth Mammalia 74 219–24
- Ungar P S, Teaford M F, Glander K E and Pastor R F 1995 Dust accumulation in the canopy: a potential cause of dental microwear in primates Am. J. Phys. Anthropol. 97 93–9
- Van Valkenburgh B and Hertel F 1993 Tough times at La Brea: tooth breakage in large carnivores of the Late Pleistocene Science 261 456–9
- Van Valkenburgh B, Teaford M F and Walker A 1990 Molar microwear and diet in large carnivores: inferences concerning diet in the sabretooth cat, Smilodon fatalis J. Zool. 222 319–40
- Walker A, Hoeck H N and Perez L 1978 Microwear of mammalian teeth as an indicator of diet Science 201 908–10
- Wang Y and Cerling T E 1994 A model of fossil tooth and bone diagenesis: implications for paleodiet reconstruction from stable isotopes Palaeogeogr. Palaeoclimtol. Palaeoecol. 107 281–9
- Wood B and Constantino P 2007 Paranthropus boisei: fifty years of evidence and analysis Am. J. Phys. Anthropol. 134 106–32
- Xia J, Zheng J, Huang D, Tian Z, Chen L, Zhou Z, Ungar P S and Qian L 2015 New model to explain tooth wear with implications for microwear formation and diet reconstruction Proc. Natl Acad. Sci. USA 112 10669–72





