Foraging ecology of ringed seals (Pusa hispida), beluga whales (Delphinapterus leucas) and narwhals (Monodon monoceros) in the Canadian High Arctic determined by stomach content and stable isotope analysis


Published: May 18, 2015
Latest article update: Aug. 20, 2023
Abstract
Stomach content and stable isotope analysis (δ13C and δ15N from liver and muscle) were used to identify habitat and seasonal prey selection by ringed seals (Pusa hispida; n=21), beluga whales (Delphinapterus leucas; n=13) and narwhals (Monodon monoceros; n=3) in the eastern Canadian Arctic. Arctic cod (Boreogadus saida) was the main prey item of all three species. Diet reconstruction from otoliths and stable isotope analysis revealed that while ringed seal size influenced prey selection patterns, it was variable. Prey-size selection and on-site observations found that ringed seals foraged on smaller, non-schooling cod whereas belugas and narwhals consumed larger individuals in schools. Further interspecific differences were demonstrated by δ13C and δ15N values and indicated that ringed seals consumed inshore Arctic cod compared to belugas and narwhals, which foraged to a greater extent offshore. This study investigated habitat variability and interseasonal variation in the diet of Arctic marine mammals at a local scale and adds to the sparse data sets available in the Arctic. Overall, these findings further demonstrate the critical importance of Arctic cod to Arctic food webs.
Keywords
Arctic marine mammals, diet, Arctic cod, stable isotopes, Bayesian mixing models, stomach contents
The value of multiple and complementary sampling techniques to study foraging behaviours and dietary preferences of Arctic marine mammals has been demonstrated in recent years (e.g., Aubail et al. 2010; Marcoux et al. 2012; Watt & Ferguson 2015). For example, stomach content analysis (SCA) provides direct identification and quantification of prey, which is essential to determine important food items (e.g., Bluhm & Gradinger 2008; Labansen et al. 2011), but can be limited by empty stomachs and unidentified food particles due to rapid digestion (Finley & Gibb 1982; Sheffield et al. 2001). Stable isotope analysis (SIA), which is based on the fact that the chemical composition of a predator reflects the chemical signature of its assimilated prey (Dalerum & Angerbjörn 2005; Newsome et al. 2010), complements SCA. Nitrogen stable isotopes (δ15N) can identify trophic position since there is a relatively predictable increase from prey to predator (3–5‰; Newsome et al. 2010), although variation in this enrichment can be significant (Vander Zanden & Rasmussen 2001). Nitrogen stable isotopes can also provide a spatial indication of feeding, such as inshore vs. offshore, because physical/biological processes such as upwelling or phytoplankton blooms can alter nitrogen isotope composition inshore (Chouvelon et al. 2012). Similarly, carbon stable isotopes (δ13C) serve as a proxy of feeding habitat because values are conserved between predator and prey (0.5–2.0‰; Newsome et al. 2010), reflecting potential sources of carbon at the base of the food web. Further, carbonates (13C) of primary producers fractionate at different rates during carbon fixation and deposition of organic materials enriches benthic carbon signals (France 1995; Vander Zanden & Rasmussen 1999). For example, benthic food webs, which are derived from algae and detritus, are generally higher in δ13C values compared to pelagic food webs, which are derived from phytoplankton (Hobson & Welch 1992; France 1995). However, a limitation of SIA is that it does not necessarily provide detailed information on the prey types consumed by predators (Hussey et al. 2013). Recently, Bayesian framework mixing models such as those in SIAR (Parnell et al. 2010) and MixSIR (Moore & Semmens 2008) have been developed to address this issue by estimating probability distributions of prey contributions based on stable isotope composition (δ15N and δ13C values) from the predator and a range of potential prey sources (Layman et al. 2012). These models directly incorporate uncertainty in multiple input parameters and different diet–tissue discrimination factors can be specified (Parnell et al. 2010). A further advantage of SIA is that, because of the time it takes for prey to be assimilated into predator tissues, the time-scale represented by SIA is greater than SCA. For example, SCA represents recent diet (hours–days) (Finley & Gibb 1982; Christiansen et al. 2005), SIA of liver provides short-term (weeks) diet assimilation information and SIA of muscle reflects medium-term diet (months) (Tieszen et al. 1983; Hobson & Clark 1992). Since all dietary techniques have inherent limitations, the combination of several in a multi-approach study improves the assessment of the animal’s feeding ecology (see Hyslop 1980; Post 2002).
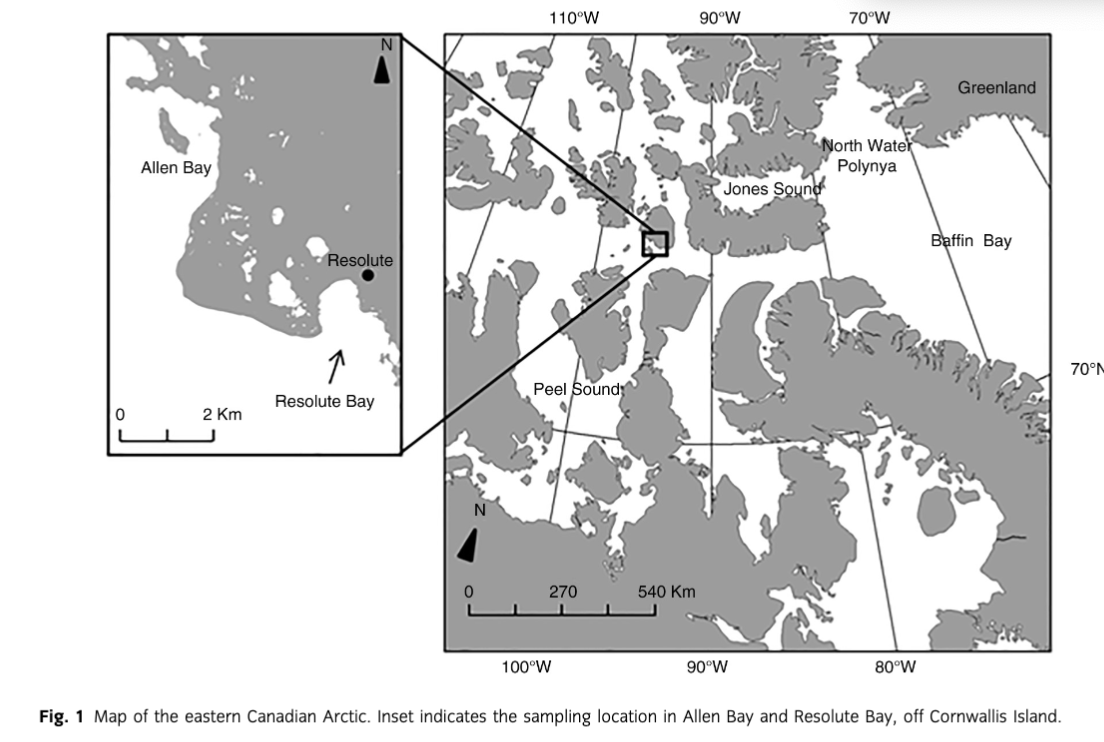
Beluga whales (Delphinapterus leucas) and narwhals (Monodon monoceros) migrate to the Canadian Arctic Archipelago during summer mainly from Baffin Bay, Jones Sound, the North Water polynya and off the western coast of Greenland (Innes et al. 1996) (Fig. 1). They typically migrate along coasts or lead in pack ice to summering areas such as Peel Sound (Mansfield et al. 1975; Richard et al. 2001), returning east as the sea ice consolidates. The summer diet of these whale species in the Canadian Arctic is dominated by Arctic cod (Boreogadus saida) (Finley & Gibb 1982; Welch et al. 1992; Loseto et al. 2009). Information about diet in wintering areas is limited but suggests that boreoatlantic armhook squid (Gonatus fabricii), capelin (Mallotus villosus) and Greenland halibut (Reinhardtius hippoglossoides) supplement Arctic cod (Heide-Jørgensen & Teilmann 1994; Laidre & Heide-Jørgensen 2005; Gardiner & Dick 2010; Watt & Ferguson 2015). Ringed seals (Pusa hispida) are Arctic and sub-Arctic phocids that typically remain in ice-covered waters year-round. Many ringed seals show area fidelity, but long-range movement and changes in seasonal distribution occur (e.g., Heide-Jørgensen et al. 1992). The diet of ringed seals in the High Arctic is dominated by only a few species, including Arctic cod, pelagic amphipods and mysids (Bradstreet & Cross 1982; Marcin 1994; Holst et al. 2001; Labansen et al. 2011).
The purpose of this study was to improve our understanding of the foraging ecology of ringed seals, belugas and narwhals using SCA and SIA in a multi-approach study. Although sampling was mainly limited to the open water period in 2010 and the size of samples was relatively small, the goal was to contribute to the sparse data sets available in the Arctic and explore potential patterns and insights into the foraging ecology of these marine mammals with which future studies could be compared. The main objectives were to: 1) determine intraspecific (ringed seal age and size comparisons) and interspecific (ringed seals vs. whales) prey selection patterns; and 2) determine the importance of prey seasonally (e.g., open water vs. ice-covered water) and spatially (e.g., inshore/benthic vs. offshore/pelagic habitat) within the Resolute and Allen Bay region using SCA and SIA of carbon and nitrogen from liver and muscle tissues.
Materials and methods
Study area
Resolute Bay and Allen Bay are located by Cornwallis Island, Nunavut, near the community of Resolute (74°41′51′′N 094°49′56′′W; Fig. 1) in the Canadian High Arctic. Belugas and narwhals routinely navigate the shores around Resolute to and from summering grounds, while ringed seals are present year-round. Resolute Bay and Allen Bay are culturally important to Inuit as the main subsistence hunting locations near Resolute. These bays are also frequented by schools of Arctic cod during the open water period and consistently attract marine mammals to the area to feed (Welch et al. 1993; Hop et al. 1997; Simeone pers. comm.).
Sample collection
Ringed seals (n=21, 2010; n=6, 2011), belugas (n=13, 2010) and narwhals (n=3, 2010) were collected as part of subsistence hunting by Inuit hunters in Resolute Bay and Allen Bay during the summer and early fall in 2010 and 2011 (Table 1). The following tissues were collected from each animal less than four hours after harvesting and frozen (−20°C): whole stomach (ringed seals: n=19, 2010; belugas: n=12; narwhals: n=3), liver and muscle (ringed seals: n=21, 2010, and n=6, 2011; belugas: n=12; narwhals: n=3), a tooth from the 2nd and 5th position on the right mandible (belugas; following Stewart et al. 2006), and lower and upper canines (ringed seals; following Stewart et al. 1996). The frozen tissue samples were later shipped to the University of Manitoba and stored at −20°C until analyses.
Table 1 Sample collection information of ringed seals, belugas and narwhals in Allen Bay and Resolute bay in 2010 and 2011. | ||||
Ringed seals | ||||
Collection date | Aug 9–Sep 14 | Jun 15–Sep 31 | Belugas Sep 1–Sep 16 | Narwhals Sep 16–Sep 17 |
Sample size (n) | 21 | 6 | 13 | 3 |
Males | 17 | 2 | 8 | 3 |
Adult | 9 | 1 | 6 | 3 |
Juvenile | 4 | 1 | 2 | – |
Pup | 4 | – | – | – |
Females | 4 | 2 | 5 | – |
Adult | 4 | 2 | 3 | – |
Juvenile | – | – | 2 | – |
Pup | – | – | – | – |
Unknown | – | 2 | – | – |
Total stomachs with contents | 17 (2)a | – | 5 (7)a | 3 (0)a |
aThe numbers in parentheses represent empty stomachs.
Age, maturity and sex determination
Ringed seals were aged by counting growth layer groups in the lower and upper canines (cementum age analysis; Stewart et al. 1996). If age (x, n=4) or length (L, n=2) was not obtained, the following growth model was used to estimate the missing value: Lx =144.5 (1−e−0.099(x+0.61))0.225 (see figure 22 in McLaren 1993). This model was selected because of the large sample size (ca. 100 ringed seals) and also because seals were sampled from the Canadian Arctic (McLaren 1993), reducing the effect of regional variability. The maturity of ringed seals was estimated after counting annual growth layers in canine teeth (Stewart et al. 1996) as follows: adult ≥6 years, juvenile 1–5 years and pup<1 year (Holst et al. 2001). Beluga age was estimated by counting annual growth layers deposited in the dentine of mounted tooth sections (Stewart 2012). Belugas were classified as adult if they were ≥12 years old (Stewart 1994; Stewart et al. 2006). When teeth were unavailable, age (x, n=2) and length (L, n=1) were estimated as follows: (female) and (male) (Luque & Ferguson 2010). The age of narwhals could not be estimated, but the lengths of their tusks (>1 m) indicated that all three were adult males (Finley & Gibb 1982). The sex of ringed seals and toothed whales was determined from a skin sample using polymerase chain reaction-based genetic analysis (Shaw et al. 2003).
Stomach content analysis
Whole stomachs were thawed then cut open, and stomach contents and epithelium were washed with water and filtered through three sieves (4 mm, 1.4 mm and 425 µm). Diet items were identified to the lowest possible taxonomic level. Sagittal otoliths showing little or no degradation were measured using an Axio Zoom V16 miscroscope and AxioCam HR camera (Zeiss, Jena, Germany) to model the fork length (FL, mm) of consumed Arctic cod. When a stomach had >25 otoliths, a subsample of 25 otoliths were measured. Otolith length (OL, mm) was defined as the longest dimension between the posterior and anterior edges of the otolith (Hunt 1992). The FL of Arctic cod was then estimated from OL using FL=24.20*OL–4.29 (r2=0.91, n=251) (Matley et al. 2013). Amphipods were enumerated by counting whole bodies and disarticulated parts—eyes and telsons (Finley & Gibb 1982).
Frequency of occurrence (FOi) and percent composition (Ni) were used to determine the importance of prey (e.g., Hyslop 1980; Pierce & Boyle 1991) as follows:
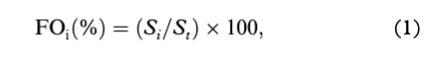
where Si represents the number of ringed seals (or belugas or narwhals) that consumed prey type i, and St represents the total number of ringed seals (or belugas or narwhals) sampled; and
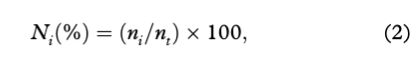
where ni represents the total number of prey type i, and nt represents the total number of prey sampled in all stomachs.
Stable isotope analysis
Liver and muscle tissue from each seal and toothed whale were analysed at the Great Lakes Institute for Environmental Research at the University of Windsor, Canada, for quantification of δ13C and δ15N. Tissues were oven-dried at 70°C for 48 h then crushed and ground. Since lipids are depleted in 13C and influence δ13C values (Caut et al. 2011), they were extracted from predator and prey tissues using a 2:1 chloroform:methanol solvent as described by McMeans et al. (2009). A continuous flow isotope ratio mass spectrometer (Finnigan MAT Deltaplus, Thermo Finnigan, San Jose, CA, USA) equipped with a Costech Elemental Analyzer (Costech Analytical Technologies, Valencia, CA, USA) determined δ13C and δ15N using Pee Dee Belemnite carbonate for CO2 and atmospheric nitrogen for N2 as standard reference materials. Stable isotope ratio values are expressed in parts per thousand (‰) using δ notation as calculated using the following equation:
where X is 15N or 13C, Rsample is the ratio (15N/14N or 13C/12C) in the sample and Rstandard is the ratio in the standard. The standard deviation of samples run in triplicate was generally <0.1‰ (δ13C) and <0.2‰ (δ15N).
Data analysis
Statistical analyses were not conducted for ringed seals in 2011 because of the small sample size (n=6), but stable isotope values are presented. Tissues from only four female ringed seals were collected in 2010 and consequently no sex comparisons were made. Similarly, the effect of life history traits on stable isotopes and stomach contents were not examined for belugas or narwhals because of small sample sizes. After preliminary data exploration showed that similar food items were consumed by belugas and narwhals, and that stable isotope values did not significantly differ between species, the toothed whales were grouped for statistical analyses. Assumptions of data normality and homogeneity of variances were verified using Q–Q plots and visual analysis of residuals, respectively. General additive modelling (GAM; Gaussian error distribution with an identity link) was used to describe how ringed seal age and length influenced the size of Arctic cod consumed. Candidate models were compared using Akaike information criterion with correction for small sample size (AICc) from the mgcv package (Wood 2000). The models with the lowest AIC values were considered to contain the most important factors influencing the size of Arctic cod consumed; however, uncertainty associated with hypothesis testing in GAMs means that this is only a general guide (e.g., Venables & Dichmont 2004). Data from one ringed seal pup which consumed Arctic cod<90 mm was omitted because it had a disproportionate influence on the output (Cook’s distance>1). The mean size of Arctic cod consumed (via SCA) by ringed seals and whales was compared with a Mann–Whitney U test.
An analysis of covariance (ANCOVA) determined the effect of maturity (juvenile/pup<6 years old, adult ≥6 years old; Holst et al. 2001) on ringed seal δ13C and δ15N values for liver and muscle with ringed seal length as the covariate. Finally, the mean liver and muscle δ15N and δ13C values were compared among ringed seals and toothed whales by analysis of variance (ANOVA), followed by a Tukey’s test. Statistical analyses were conducted with the R program (R Core Team 2013), and differences p≤0.05 were considered statistically significant.
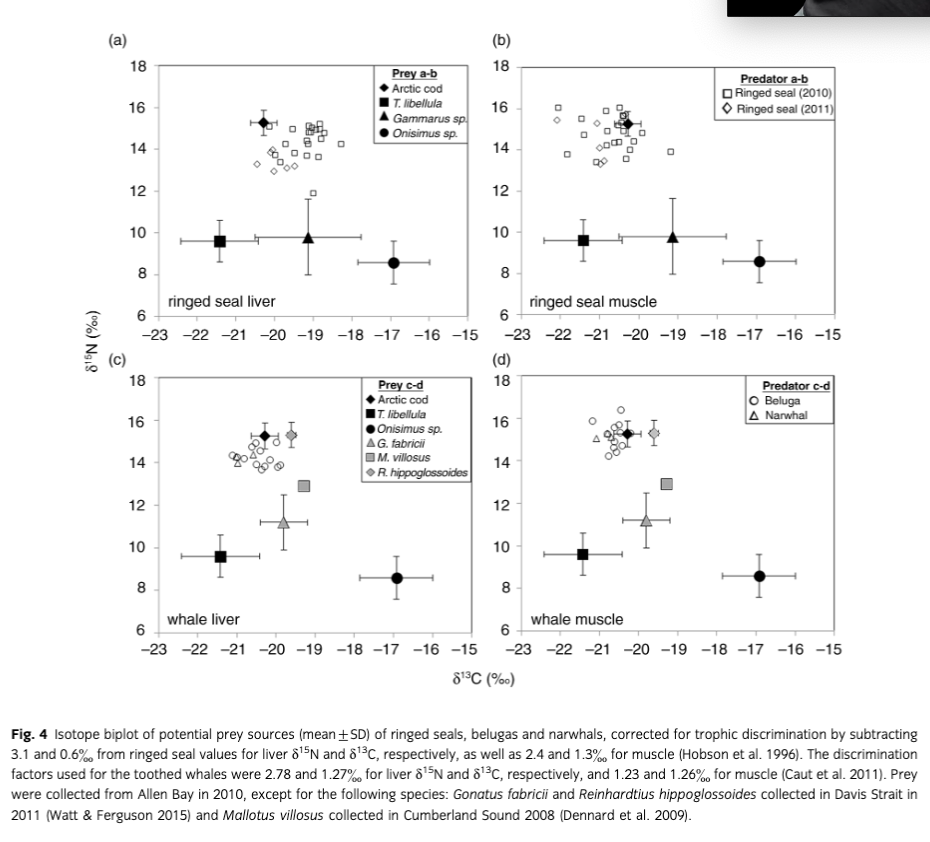
To quantify the importance of potential prey of ringed seals in 2010 near Resolute, the Bayesian mixing model SIAR (SIA in R) from the SIAR package (Parnell et al. 2010) was used with diet–tissue discrimination factors estimated from harp seals (Pagophilus groenlandicus) by Hobson et al. (1996) (muscle: Δ15n=2.4, Δ13C=1.3; liver: Δ15n=3.1, Δ13C=0.6). An arbitrary standard deviation of 0.2 was selected for each discrimination factor similar to bowhead whale (Balaena mysticetus) skin–prey isotopic discrimination estimates (Pomerleau et al. 2012). Stable isotope values (mean±SD) of common prey also collected in Allen Bay between July and August 2010 (see Matley et al. 2013) were used to estimate prey proportion as follows: Arctic cod (muscle: δ15N=15.27±0.61, δ13C=−20.28±0.34); Themisto libellula (δ15N=9.6±1.00, δ13C=−21.42±1.00); Onisimus sp. (δ15N=8.58±1.02, δ13C=−16.92±0.93); and Gammarus sp. (δ15N=9.80±1.83, δ13C=−19.13±1.37). These prey only represent a subset of potential food items of ringed seals in the High Arctic; however, only prey found in SCA were included to reduce contribution of non-prey items to the output. Also, Arctic cod were the focus as they are a dominant biomass in the Arctic Ocean and are well known to be very important in the diet of the marine mammals examined. Mixing models were also completed for toothed whales using discrimination factors estimated by Caut et al. 2011 (muscle: Δ15N=1.23, Δ13C=1.26; liver: Δ15N=2.78, Δ13C=1.27). Additional prey items were explored to account for dietary changes during seasonal migrations, including Gonatus fabricii (δ15N=11.2±1.3, δ13C=−19.8±0.6; Watt & Ferguson 2015), Reinhardtius hippoglossoides (δ15N=15.3±0.6, δ13C=−19.6±0.1; Watt & Ferguson 2015) and Mallotus villosus (δ15N=12.9±0.1, δ13C=−19.3±0.1; Dennard et al. 2009). Isotope values of Arctic cod and R. hippoglossoides were nearly identical, exceeding 90% in the correlation matrix, which made it difficult to differentiate the contribution of the two prey sources. Consequently, only values from Arctic cod were used but the final output is representative of possible contribution from both prey. Isotope values from prey and predators were also plotted after correcting for discrimination factors (described above) to visualize potential prey sources.
Results
Stomach contents
Forty-two percent of belugas, 89% of ringed seals and all narwhals had food in stomachs (Table 1). Arctic cod measuring between 160 and 200 mm and amphipods (Themisto libellula, Onisimus sp. and Gammarus sp.) were the main prey species (Fig. 2, Table 2). Results from the GAM demonstrated that age had greater importance describing the size of Arctic cod consumed by ringed seals (Fig. 3, Table 3); however, the small sample size led to considerable variability in the output and large confidence intervals. The mean length (±SE) of Arctic cod consumed by ringed seals (168.0±4.3 mm) was significantly smaller than the toothed whales (182.5±2.0 mm) (Mann–Whitney U test, W=21, P=0.01; Fig. 2.
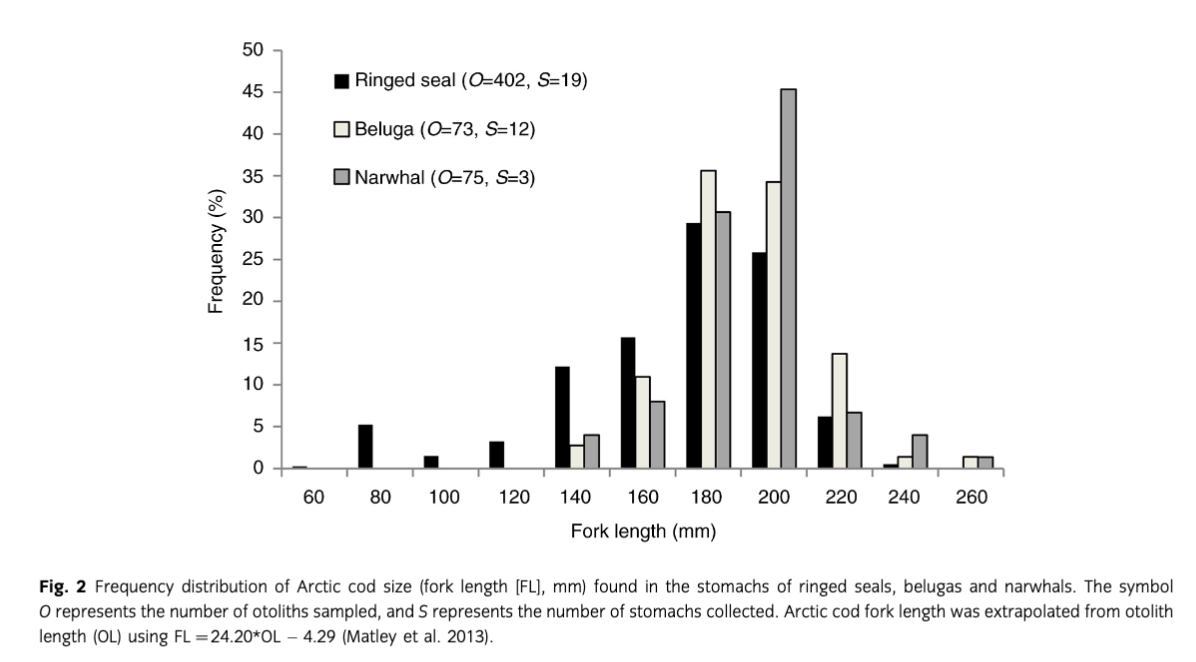
Table 2 Number of prey, frequency of occurrence (FOi) and percent composition (Ni) of ringed seal, beluga and narwhal food items. | |||
Number of preya | FOi | Ni | |
Ringed seals | |||
Juveniles and pups (n=8) | |||
Arctic cod | 160 | 87.5 | 23.6 |
Amphipods | 519b | 50 | 76.4 |
Adult (n=11) | |||
Arctic cod | 578 | 100 | 91.9 |
Amphipods | 51 | 36.4 | 8.1 |
Belugas (n=12) | |||
Arctic cod | 556 | 41.7 | 93.6 |
Amphipods | 34 | 16.7 | 5.7 |
Squid | 4 | 25 | <1 |
Narwhals (n=3) | |||
Arctic cod | 257 | 100 | 81.6 |
Amphipods | 55 | 66.7 | 17.5 |
Squid | 3 | 66.7 | 1 |
aNumber of Arctic cod calculated by dividing the total number of otoliths by 2 (e.g., 1476/2=738 individuals). bTwo individuals (a juvenile and a pup) contributed ca. 98% of amphipods collected.
Table 3 Model selection from generalized additive modelling (GAM) examining the effect of ringed seal age and length on the size of consumed Arctic cod (FL). Akaike information criterion accounting for small sample size (AICc) was used to select the best models (i.e., lower AICc), and the relative importance of each model—its weight—is indicated. | ||||
Model | AICc | ΔAICc | Weight | |
M1 | FL~s(Age) | 140.3 | 0 | 0.52 |
M2 | FL~s(Length) | 141.8 | 1.52 | 0.25 |
M3 | FL~s(Age) + s(Length) | 142.9 | 2.64 | 0.14 |
M4 | FL~1 (null model) | 143.8 | 3.57 | 0.09 |
Stable isotopes
Tissue values of δ13C were similar among predators except in the livers of ringed seals, which were significantly higher than all other samples (ANOVA, F3,68=12.44, P<0.01; Table 4. Muscle δ15N values from the toothed whales were significantly lower than δ15N from ringed seal liver and muscle (ANOVA, F3,68=7.97, p<0.01; Table 4). Ringed seal δ15N and δ13C values for liver and muscle did not differ between adults and juveniles when length was included as a covariate (Table 5). Liver δ15N was positively related to length (ANCOVA, F1,18=5.80, p=0.03;Table 5).
Table 4 Sample number (n), mean±1 standard error (SE) of liver and muscle δ15N (‰) and δ13C (‰) of ringed seals, belugas and narwhals collected near Resolute in 2010 and 2011. | |||||
Liver | Muscle | ||||
Species | n | δ15N | δ13C | δ15N | δ13C |
Ringed seals (2010) | 21 | 17.45±0.18 | −18.56±0.10 | 17.21±0.18 | −19.35±0.14 |
Ringed seals (2011) | 6 | 16.50±0.17 | −19.37±0.14 | 16.96±0.43 | −19.76±0.23 |
Belugas | 12 | 17.00±0.12 | −19.14±0.10 | 16.33±0.20 | −19.28±0.09 |
Narwhals | 3 | 16.97±0.12 | −19.57±0.14 | 16.34±0.07 | −19.59±0.11 |
Table 5 Summary of ANCOVA results testing the effect of ringed seal maturity (adult ≥6 years old, juvenile/pup < 6 years old) and length (covariate) on muscle and liver δ13C and δ15N values. Significant relationships (p≤0.05) are in boldface. | |||
Parameter | F value | P value | |
Muscle δ13C | Length | <0.01 | 0.93 |
Maturity | <0.01 | 0.99 | |
Muscle δ15N | Length | 3.86 | 0.07 |
Maturity | 1.12 | 0.30 | |
Liver δ13C | Length | 0.22 | 0.64 |
Maturity | <0.01 | 0.98 | |
Liver δ15N | Length | 5.80 | 0.03 |
Maturity | 1.22 | 0.29 | |
After correcting for diet–tissue discrimination factors, the biplot of predator and prey isotopes showed that ringed seal and whale tissues were closely associated with Arctic cod (Fig. 4). Similarly, the stable isotope mixing model estimated that Arctic cod was the main contributor to the diet of ringed seals in liver and muscle tissues (95% credibility interval: liver=65–85%; muscle=84–96%; Fig. 5a, b). The sympagic (ice-associated) amphipod Onisimus sp. was also important in liver tissue of ringed seals (95% credibility interval: 11–31%; Fig. 5a). For the toothed whales, Arctic cod and/or Reinhardtius hippoglossoides were the main prey (95% credibility interval: liver=67–83%; muscle=83–97%; Fig. 5c, d).

Discussion
Arctic cod was the dominant prey item of ringed seals, narwhals and belugas collected from the Resolute Bay region during the open water period. This is not surprising considering that Arctic cod aggregated in abundant schools in Allen Bay during the sampling period (see Matley et al. 2012) and are known to be heavily exploited by predators in this area (Hobson & Welch 1992; Hop et al. 1997). This research also showed differences in ringed seal feeding patterns among individuals and compared to the toothed whales based on multiple sampling techniques.
Ringed seal life history traits (age and length) did not have a clear influence on the size of Arctic cod consumed or muscle stable isotope (δ15N and δ13C) values, although a larger sample size is needed to investigate this further. Similarly, Holst et al. (2001) found no difference in the size of Arctic cod consumed between age classes and between sexes at two High Arctic locations. Also, Dehn et al. (2007) did not find a difference between muscle δ15N and δ13C with age of ringed seals in the Canadian Arctic, and suggested that a mixture of krill and gadid fish comprised ringed seal diet independent of life history. Liver δ15N values of ringed seals were influenced by length likely because the smaller individuals were eating smaller prey such as amphipods in addition to Arctic cod during the open water period, as supported by SCA. Higher digestion rates of smaller prey may have resulted in overestimation of Arctic cod in the diet, but the isotopic mixing model is consistent with SCA findings.
The energetic importance of Arctic cod as prey is well documented in the Arctic food web, and mainly derives from large, dense and readily visible aggregations exploited by seabirds, seals and whales near the surface (Welch et al. 1993; Crawford & Jorgenson 1996). However, smaller and sparsely dispersed non-schooling Arctic cod appear to be a significant source of food, particularly for ringed seals. During several months of observations in Allen Bay (Matley et al. 2012), ringed seals did not forage on schools of Arctic cod near the surface, yet belugas and harp seals often did—an observation also noted by Bradstreet et al. (1986). Interestingly, the mean length of net-captured Arctic cod from schools in Allen Bay was 187.0 mm (Crawford & Jorgenson 1996), while non-schooling adults in Resolute Bay were 163.5 mm (Hop et al. 1997). Therefore, it appears that ringed seals consumed non-schooling Arctic cod (162.1 mm), and toothed whales exploited Arctic cod schools (183.1 and 181.4 mm, respectively). However, the low number of predator samples available necessitates further studies to validate this preliminary finding. Further differences in feeding patterns between ringed seals and toothed whales were identified from SIA. For example, δ13C and δ15N values from ringed seals were typically higher than values from belugas and narwhals. Considering that Arctic cod were the main prey for all tissues and species, these differences may represent isotopic variation in feeding habitat and not necessarily trophic structure. Both δ13C and δ15N values are often higher in inshore/benthic habitats compared to offshore/pelagic on account of processes associated with differential fractionation (Vander Zanden & Rasmussen 2001), benthic–pelagic coupling (Hobson et al. 1995) and other physical processes, such as the demand/uptake of dissolved inorganic nitrogen (Montoya 2007; Chouvelon et al. 2012). Therefore, higher values of both δ13C and δ15N in the tissues of ringed seals support foraging in coastal areas (e.g., Teilmann et al. 1999), whereas the male-biased toothed whale tissues indicated that foraging occurred mainly offshore in deeper water (e.g., Loseto et al. 2009). Alternatively, variation in baseline δ15N and δ13C values associated with distribution patterns—migratory whales and more resident ringed seals—may have contributed to interspecific isotope patterns (e.g., Ruiz-Cooley et al. 2012).
Liver and muscle δ13C and δ15N values were similar for the toothed whales indicating that prey selection and feeding habitat were consistent over a temporal scale of months. The mixing model for the toothed whale data also supported this finding. However, the relative contribution of Arctic cod/Reinhardtius hippoglossoides was difficult to differentiate. Nevertheless, R. hippoglossoides has not been documented near Resolute either by SCA (e.g., Welch et al. 1992) or fishing (e.g., Crawford & Jorgenson 1996; Kessel, pers. comm.) and is unlikely to contribute to the tissue isotope values of predators in this area. Seasonal trends were not detected from skin and muscle δ13C and δ15N values of belugas sampled in Cumberland Sound (Marcoux et al. 2012). However, seasonal differences (as indicated by skin and muscle δ13C and δ15N values) were identified in Baffin Bay narwhals, where the contribution of capelin to the diet appeared to differ between winter and spring (Watt & Ferguson 2015).
Understanding the trophic ecology of migratory animals such as belugas and narwhals is difficult as large-scaled sampling is required to account for prey availability and baseline isotopic differences (e.g., Ruiz-Cooley et al. 2012) throughout geographic distributions. Additional caveats associated with dietary sampling make matters more difficult. For example, diet–tissue discrimination factors and turnover rates are largely unknown for marine mammals (Newsome et al. 2010). Nevertheless, our results are interesting in that diet and habitat of both belugas and narwhals were similar along migratory routes. Further, dietary similarities between belugas and narwhals indicated by SCA and SIA suggest that both species share a trophic niche, at least in the Resolute and Allen Bay area, which could have important conservation implications.

This study used SIA for liver and muscle tissues from three Arctic marine mammals in conjunction with SCA, to investigate how diet, prey composition and habitat vary seasonally around Resolute Bay and Allen Bay. While sample sizes were small these preliminary data have provided new insights into feeding patterns from traditionally harvested animals that are impossible to acquire through large-scale scientific collections in the High Arctic today. Further, this research provides baseline prey selection patterns, at a local scale, from a highly valued traditional resource use area in the Canadian Arctic. It also highlights the ecological significance of Arctic cod.
Acknowledgements
We thank S. Simeone and B. Iqaluk from Resolute for assistance collecting samples, A. Hussey for processing samples for stable isotopes and A. Sett, X. Wang and D. Muir for additional samples. We would also like to thank K. Gardiner, O. Friesen and J. Roth for advice and technical support, B. Stewart and L. Loseto for beluga ageing and D. Tenkula and L. Postma for DNA analysis. This research would not have been possible without the use of the Polar Continental Shelf Base and their staff, including M. Bergmann, Y. Laroche, B. Eckalook, J. MacGregor and M. Kristjanson, as well as the Hunters and Trappers Organization of Resolute Bay. At the time this research was carried out, JKM was at the Department of Biological Sciences, University of Manitoba. Funding was provided by the Canadian Foundation for Innovation, the Natural Sciences and Engineering Research Council, in association with the Ocean Tracking Network to TAD and ATF. We acknowledge the Kenneth M. Molson Foundation and the Faculty of Science at the University of Manitoba for additional funding. Finally, we thank the reviewers for their comments that have greatly improved this article.
References
- Aubail A., Dietz R., Rigét F., Simon-Bouhet B. & Caurant F. 2010. An evaluation of teeth of ringed seals (Phoca hispida) from Greenland as a matrix to monitor spatial and temporal trends of mercury and stable isotopes. Science of the Total Environment 408, 5137–5146. Publisher Full Text
- Bluhm B.A. & Gradinger R. 2008. Regional variability in food availability for Arctic marine mammals. Ecological Applications 18, S77–S96. Publisher Full Text
- Bradstreet M.S.W. & Cross W.E. 1982. Trophic relationships at High Arctic ice edges. Arctic 35, 1–12.
- Bradstreet M.S.W., Finley K.J., Sekerak A.D., Griffiths W.B., Evans C.R., Fabijan M.F. & Stallard H.E. 1986. Aspects of the biology of cod (Boreogadus saida) and its importance in Arctic marine food chains. Canadian Technical Report of Fisheries and Aquatic Sciences 1491. Winnipeg: Fisheries and Oceans, Canada.
- Caut S., Laran S., Garcia-Hartmann E. & Das K. 2011. Stable isotopes of captive cetaceans (killer whales and bottlenose dolphins). The Journal of Experimental Biology 214, 538–545. Publisher Full Text
- Chouvelon T., Spitz J., Caurant F., Chappuis A., Laugier F., Le Goff E. & Bustamante P. 2012. Revisiting the use of δ15N in meso-scale studies of marine food webs by considering spatio-temporal variations in stable isotopic signatures—the case of an open ecosystem: the Bay of Biscay (north-east Atlantic). Progress in Oceanography 101, 95–102. Publisher Full Text
- Christiansen J.S., Gamst Moen A-G., Hansen T.H. & Nilssen K.T. 2005. Digestion of capelin, Mallotus villosus (Müller), herring, Clupea harengus L., and polar cod, Boreogadus saida (Lepechin), otoliths in a simulated seal stomach. ICES Journal of Marine Science 62, 86–92. Publisher Full Text
- Crawford R.E. & Jorgenson J.K. 1996. Quantitative studies of cod (Boreogadus saida) schools: important energy stores in the Arctic food webs. Arctic 49, 181–193. Publisher Full Text
- Dalerum F. & Angerbjörn A. 2005. Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia 144, 647–658. Publisher Full Text
- Dehn L.A., Sheffield G.G., Follmann E.H., Duffy L.K., Thomas D.L. & O’Hara T.M. 2007. Feeding ecology of phocid seals and some walrus in the Alaskan and Canadian Arctic as determined by stomach contents and stable isotope analysis. Polar Biology 30, 167–181. Publisher Full Text
- Dennard S.T., McMeans B.C. & Fisk A.T. 2009. Preliminary assessment of Greenland halibut diet in Cumberland Sound using stable isotopes. Polar Biology 32, 941–945. Publisher Full Text
- Finley K.J. & Gibb E.J. 1982. Summer diet of the narwhal (Monodon monoceros) in Pond Inlet, northern Baffin Island. Canadian Journal of Zoology 60, 3353–3363. Publisher Full Text
- France R.L. 1995. Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Marine Ecology Progress Series 124, 307–312. Publisher Full Text
- Gardiner K. & Dick T.A. 2010. Arctic cephalopod distributions and their associated predators. Polar Research 29, 209–227. Publisher Full Text
- Heide-Jørgensen M.P., Stewart B.S. & Leatherwood S. 1992. Satellite tracking of ringed seals Phoca hispida off northwest Greenland. Ecography 15, 56–61. Publisher Full Text
- Heide-Jørgensen M.P. & Teilmann J. 1994. Growth, reproduction, age structure and feeding habits of white whales (Delphinapterus leucas) in west Greenland waters. Bioscience 39, 195–212.
- Hobson K.A., Ambrose W.G. Jr., & Renaud P.E. 1995. Sources of primary production, benthic–pelagic coupling, and trophic relationships with the Northeast Water Polynya: insights from 13C and 15N analysis. Marine Ecology Progress Series 128, 1–10. Publisher Full Text
- Hobson K.A. & Clark R.G. 1992. Assessing avian diets using stable isotopes II: factors influencing diet–tissues fractionation. Condor 94, 189–197. Publisher Full Text
- Hobson K.A., Schell D.M., Renouf D. & Noseworthy E. 1996. Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Canadian Journal of Fisheries and Aquatic Sciences 53, 528–533. Publisher Full Text
- Hobson K.A. & Welch H.E. 1992. Determination of trophic relationships within a High Arctic marine food web using δ13C and δ15N analysis. Marine Ecology Progress Series 84, 9–18. Publisher Full Text
- Holst M., Stirling I. & Hobson K.A. 2001. Diet of ringed seals (Phoca hispida) on the east and west sides of the North Water Polynya, northern Baffin Bay. Marine Mammal Science 17, 888–908. Publisher Full Text
- Hop H., Welch H.E. & Crawford R.E. 1997. Population structure and feeding ecology of cod (Boreogadus saida) schools in the Canadian High Arctic. In J. Reynolds (ed.): Fish ecology in Arctic North America. Pp. 68–80. Bethesda: American Fisheries Society.
- Hunt J.J. 1992. Morphological characteristics of otoliths for selected fish in the northwest Atlantic. Journal of Northwest Atlantic Fishery Science 13, 63–75. Publisher Full Text
- Hussey N.E., MacNeil M.A., McMeans B.C., Olin J.A., Dudley S.F.J., Cliff G., Wintner S.P., Fennessy S.T. & Fisk A.T. 2013. Rescaling the trophic structure of marine food webs. Ecology Letters 17, 239–50. Publisher Full Text
- Hyslop E.J. 1980. Stomach contents analysis—a review of methods and their application. Journal of Fish Biology 17, 411–429. Publisher Full Text
- Innes S., Heide-Jørgensen M.P., Laake J.L., Laidre K.L., Cleator H.J., Richard P. & Stewart R.E.A. 1996. Surveys of belugas and narwhals in the Canadian High Arctic in 1996. NAMMCO Scientific Publications 4, 169–190. Publisher Full Text
- Labansen A.L., Lydersen C., Levermann N., Haug T. & Kovacs K.M. 2011. Diet of ringed seals (Pusa hispida) from northeast Greenland. Polar Biology 34, 227–234. Publisher Full Text
- Laidre K. & Heide-Jørgensen M.P. 2005. Winter feeding intensity of narwhals (Monodon monoceros). Marine Mammal Science 21, 45–57. Publisher Full Text
- Layman C.A., Araujo M.S., Boucek R., Hammerschlag-Peyer C.M., Harrison E., Jud Z.R., Matich P., Rosenblatt A.E., Vaudo J.J., Yeager L.A., Post D.M. & Bearhop S. 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological Reviews 87, 545–562. Publisher Full Text
- Loseto L., Stern G., Connelly T., Deibel D., Gemmill B. & Prokopowicz A. 2009. Summer diet of beluga whales inferred by fatty acid analysis of the eastern Beaufort Sea food web. Journal of Experimental Marine Biology and Ecology 374, 12–18. Publisher Full Text
- Luque S.P. & Ferguson S.H. 2010. Age structure, growth, mortality, and density of belugas (Delphinapterus leucas) in the Canadian Arctic: responses to environment? Polar Biology 33, 163–178. Publisher Full Text
- Mansfield A.W., Smith T.G. & Beck B. 1975. Narwhal, Monodon monoceros, in eastern Canadian waters. Journal of the Fisheries Research Board of Canada 32, 1041–1046. Publisher Full Text
- Marcin W.J. 1994. Diet of ringed seals (Phoca hispida) in a fjord of West Svalbard. Arctic 47, 109–114.
- Marcoux M., McMeans B.C., Fisk A.T. & Ferguson S.H. 2012. Composition and temporal variation in the diet of beluga whales, derived from stable isotopes. Marine Ecology Progress Series 47, 283–291. Publisher Full Text
- Matley J.K., Fisk A.T. & Dick T.A. 2012. Seabird predation on Arctic cod during summer in the Canadian Arctic. Marine Ecology Progress Series 450, 219–228. Publisher Full Text
- Matley J.K., Fisk A.T. & Dick T.A. 2013. The foraging ecology of Arctic cod (Boreogadus saida) during open water (July–August) in Allen Bay, Arctic Canada. Marine Biology 160, 2993–3004. Publisher Full Text
- McLaren I.A. 1993. Growth in pinnipeds. Biological Reviews of the Cambridge Philosophical Society 68, 1–79. Publisher Full Text
- McMeans B.C., Olin J.A. & Benz G.W. 2009. Stable-isotope comparisons between embryos and mothers of a placentatrophic shark species. Journal of Fish Biology 75, 2464–2474. Publisher Full Text
- Moore J.W. & Semmens B.X. 2008. Incorporating uncertainty and prior information in stable isotope mixing models. Ecological Letters 11, 470–480. Publisher Full Text
- Montoya J.P. 2007. Natural abundance of 15N in marine planktonic ecosystems. In R. Michener & K. Lajtha (eds.): Stable isotopes in ecology and environmental science. 2nd edn. Pp. 176–201. Malden, MA: Blackwell.
- Newsome S.D., Clementz M.T. & Koch P.L. 2010. Using stable isotope biogeochemistry to study marine mammal ecology. Marine Mammal Science 26, 509–572.
- Parnell A.C., Inger R., Bearhop S. & Jackson A.L. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS One 5, e9672, doi 10.1371/journal.pone.0009672. Publisher Full Text
- Pierce G.J. & Boyle P.R. 1991. A review of methods for diet analysis in piscivorous marine mammals. Oceanography and Marine Biology: Annual Review 29, 409–486.
- Pomerleau C., Lesage V., Ferguson S.H., Winkler G., Petersen S.D. & Higdon J.W. 2012. Prey assemblage isotopic variability as a tool for assessing diet and the spatial distribution of bowhead whale Balaena mysticetus foraging in the Canadian eastern Arctic. Marine Ecology Progress Series 469, 161–174. Publisher Full Text
- Post D.M. 2002. Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83, 703–718.
- R Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Richard P.R., Heide-Jørgensen M.P., Orr J.R., Dietz R. & Smith T.G. 2001. Summer and autumn movements and habitat use by belugas in the Canadian High Arctic and adjacent areas. Arctic 54, 207–222.
- Ruiz-Cooley R.I., Engelhaupt D.T. & Ortega-Ortiz J.G. 2012. Contrasting C and N isotope ratios from sperm whale skin and squid between the Gulf of Mexico and Gulf of California: effect of habitat. Marine Biology 159, 151–164. Publisher Full Text
- Shaw C.N., Wilson P.J. & White B.N. 2003. A reliable method of gender determination for mammals. Journal of Mammalogy 84, 123–128. Publisher Full Text
- Sheffield G., Fay F.H., Feder H. & Kelly B.P. 2001. Laboratory digestion of prey and interpretation of walrus stomach contents. Marine Mammal Science 17, 310–330. Publisher Full Text
- Stewart B.E. 2012. A technical report on methods for tooth preparation and age estimates of beluga (Delphinapterus leucas). Canadian Technical Report of Fisheries and Aquatic Sciences 3020. Winnipeg: Fisheries and Oceans Canada.
- Stewart R.E.A. 1994. Size-at-age relationships as discriminators of white whale (Delphinapterus leucas) stocks in the eastern Canadian Arctic. Bioscience 39, 217–225.
- Stewart R.E.A., Campana S.E., Jones C.M. & Stewart B.E. 2006. Bomb radiocarbon dating calibrates beluga (Delphinapterus leucas) age estimates. Canadian Journal of Zoology 84, 1840–1852. Publisher Full Text
- Stewart R.E.A., Stewart B.E., Stirling I. & Street E. 1996. Counts of growth layer groups in cementum and dentine in ringed seals (Phoca hispida). Marine Mammal Science 12, 383–401. Publisher Full Text
- Teilmann J., Born E.W. & Acquarone M. 1999. Behaviour of ringed seals tagged with satellite transmitters in the North Water polynya during fast-ice formation. Canadian Journal of Zoology 77, 1934–1946. Publisher Full Text
- Tieszen L.L., Boutton T.W., Tesdahl K.G. & Slade N.A. 1983. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57, 32–37. Publisher Full Text
- Vander Zanden M.J. & Rasmussen J.B. 1999. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80, 1395–1404.
- Vander Zanden M.J. & Rasmussen J.B. 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 48, 2061–2066. Publisher Full Text
- Venables W.N. & Dichmont C.M. 2004. GLMs, GAMs and GLMMs: an overview of theory for applications in fisheries research. Fisheries Research 70, 319–337. Publisher Full Text
- Watt C.A. & Ferguson S.H. 2015. Fatty acids and stable isotopes (δ13C and δ15N) reveal temporal changes in narwhal (Monodon monoceros) diet linked to migration patterns. Marine Mammal Science 31, 21–44. Publisher Full Text
- Welch H.E., Bergmann M.A., Siferd T.D., Martin K.A., Curtis M.F., Crawford R.E., Canover R.J. & Hop H. 1992. Energy flow through the marine ecosystem of the Lancaster Sound region, Arctic Canada. Arctic 45, 343–357. Publisher Full Text
- Welch H.E., Crawford R.E. & Hop H. 1993. Occurrence of cod (Boreogadus saida) schools and their vulnerability to predation in the Canadian High Arctic. Arctic 46, 331–339.
- Wood S.N. 2000. Modelling and smoothing parameter estimation with multiple quadratic penalties. Journal of the Royal Statistical Society Series B 62, 413–428. Publisher Full Text





