Improvement in cocoon yield induced by phytojuvenoid on the multivoltine mulberry silkworm (Bombyx mori Linn.)


Published: Jan. 30, 2015
Latest article update: Aug. 21, 2022
Abstract
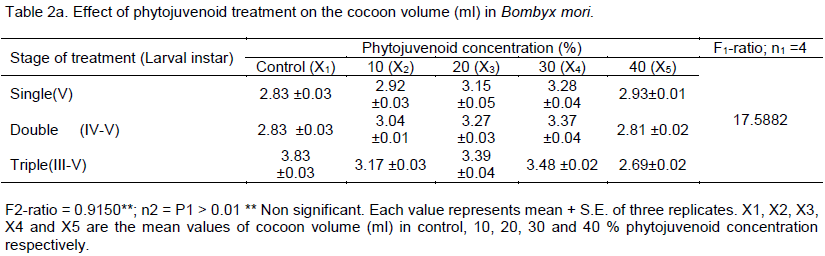
Impact of phytojuvenoid on commercial parameters in Bombyx mori, a monophagous insect, was studied. The variation in the phytojuvenoid concentration significantly influenced the length of cocoon and the number of larval treatment did not cause significant influence on the length of cocoon of B. mori. The length of cocoon increased from 2.88 cm (control) to the maximum level of 3.65 cm in 30% phytojuvenoid concentration - triple treated larvae and the volume of cocoon increased with increasing the number of larval treatment from single to triple in 10, 20 and in 30% phytojuvenoid concentration and the volume was highest (3.48 ml) in 30% phytojuvenoid concentration at triple treated larvae. The results show that topical application of bioactive phytojuvenoid improved the commercial parameters in B. mori.
Keywords
Larval treatment., Bombyx mori, larvae, Phytojuvenoid, silk producing potential
INTRODUCTION
Silk, the natural fiber that spells splendor lusture and elegance, has been an inseparable part of Indian culture and tradition, over thousands of years. Mulberry sericulture in India is a commercially attractive and sustainable farm based economic enterprise positively favoring the rural poor in the unorganized sector. Nistari is a resistant variety of multivoltine mulberry silkworm (Bombyx mori) which contributes up to a great extent in the commercial production of cocoon. The efforts are being made to evolve new technologies that are effective, labour saving and eco-friendly. In order to increase, the production of silk, efforts have been made to study effect of ecological factor, photoperiod, artificial diet (Iwanvat and Ono, 1969), X-rays (Kanarev and Cham, 1985) etc. on the performance of silkworm. The Magnetization of eggs influences silk producing potential and incubation period of eggs (Upadhyay and Prasad, 2010b) and larval performance (Prasad and Upadhyay, 2011).
The phytoecdysteroid has been noticed to influence the development, growth, silk producing and reproductive potential of B. mori (Srivastava and Upadhyay, 2012a, b). The juvenile hormone (JH) analogue also has been noticed to influence the reproductive and commercial potential of B. mori (Srivastava and Upadhyay, 2013a, b, c; Nair et al., 2003). The JH analogues and mimics have been reported to have some hormonal influence on the growth of B. mori and cocoon production (Nair et al., 2006). However, the response to such treatment varies depending on the dosage of compounds showing duration and number of applications (Chowdhary et al., 1990).
The more food ingested during this period, the more it gets converted and in turn contributes to silk protein. Delay in moulting is probably due to the inhibitory action of JH on ecdysone synthesis in B.mori (Sakurai et al., 1986; Trivedy et al., 1997). JH is claimed to inhibit protein synthesis in early treated larvae with later on region protein synthesis resulting in bigger silk gland and the result is improvement of cocoon shell weight (Garel, 1983). Some plants like Pinus longifolia, Abies balsomea, Psorelea corylifolia and Azadiracta indica act on B. mori larvae as bioactive juvenoid compounds (Nair et al., 1999). Keeping this in view, an attempt has been made to study the topical effect of bioactive phytojuvenoid on the improvement in the commercial parameters in this monphagous insect (B. mori), which is the aim of the present investigation.
MATERIALS AND METHODS
RESULTS
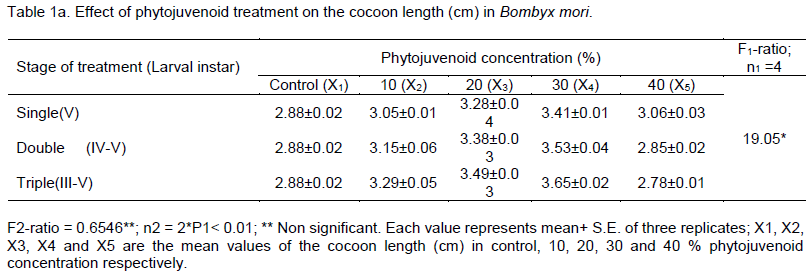
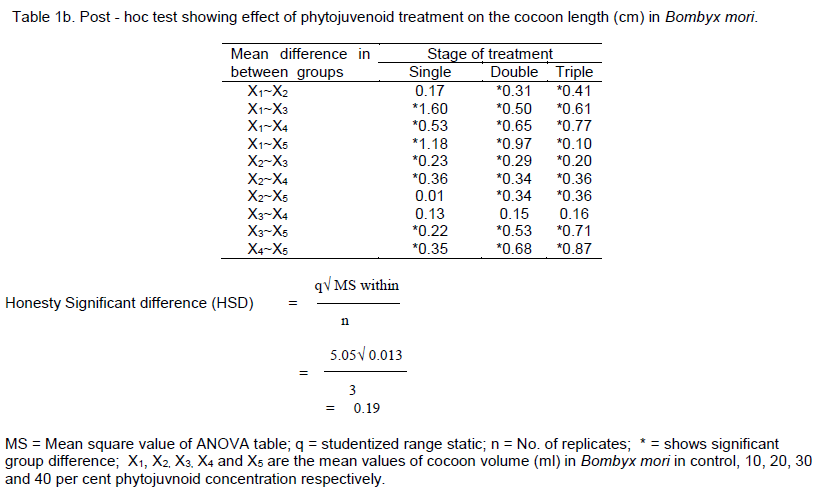
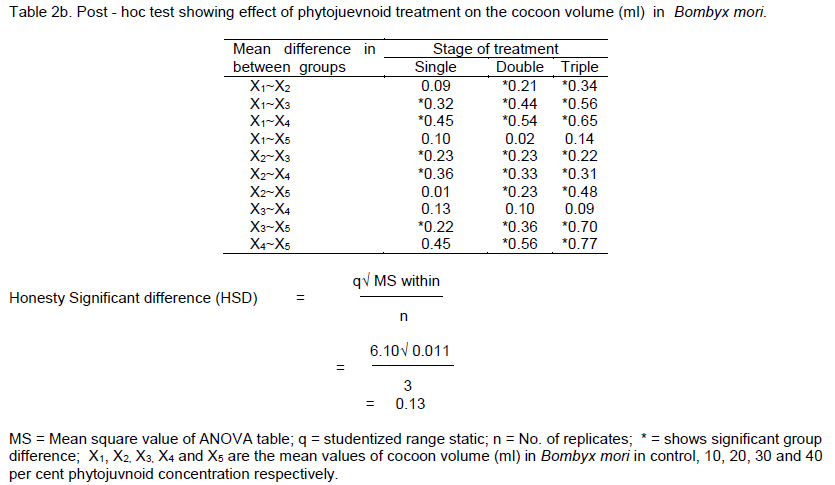
DISCUSSION
CONFLICT OF INTERESTS
The author(s) have not declared any conflict of interests.
REFERENCES
Akai H, Kiguchi K, Mox K (1971). Increased accumulation of silk protein accompanies JH-induced prolongation of larval life in Bombyx mori. Appl. Entomol. Zool. 6:218-220. | |
| |
Chowdhary SK, Raju PS, Ogra RK (1990). Effects of JH analogues on silkworm, Bombyxmori L. growth and development of silk gland. Sericolgia 30(2):155-165. | |
| |
Gamo T, Satio S, Ohtsuka Y, Hirobe T, Tazima Y (1985). Estimation of combining ability and genetic analysis by dialed cross between regional races of silkworm (2) shape and size of cocoons. Tech. Bull. Seric. Exp. Stn. 126:121-135. | |
| |
Garel JP (1983). The physiology and biology of spinning in Bombyx mori V Endocrinologcal aspects of silk production. Experientia, 39(5):461-466. | |
| |
Iwanvat Y, Ono Y (1969). Rearing experiments on artificial diet composed mainly on the mulberry beat powder harvested in the late autumn and after. J. Seric. Sci. 38:307-315. | |
| |
Jolly MS (1983). Organization of industrial bivoltinegrainage for tropics. Seric. Project No. 3. C.S.R. and T.I. Mysore, Govt. of India, 19-20. | |
| |
Kanarev G, Cham GT (1985). Effect of laser irradiation of silkworm eggs on silkworm Bombyx mori development and productivity. Zhivotnov Nauki 22:47-53. | |
| |
Krishnaswamy S, Narasimhanna MM, Suryanaryan SK, Kumar RS (1973) Sericulture Manual 2. Silkworm Rearing. F.A.O.Agric. Serves Bull. Rome. 15 (2):1-131. | |
| |
Nair KS, Morison MN, Nirmal KSM, Sabita N (2006) Impact of hormonally induced larval maturation and uniform spinning on the cocoon and grainage traits of pure race of bivoltine silkworm Bombyx mori L. Indian J. Seric. 45(1):45-50. | |
| |
Nair KS, Nair JS, Trivedy K, Vijayan VA (2003). Influence of bakuchiol, a JH analogue from bemchi (Psoraleacorylifolia) on silk production in silkworm, Bombyx mori L (Bombycidae:Lepidoptera). J. Appl. Sci. Environ. Manage., 7:31-38. | |
| |
Nair KS, Nair JS, Vijayan VA, Trivedy K (1999). Juvenilomimic compounds for enhance productivity in silkworm, Bombyx mori - A screening. Indian J. Seric. 38(2):119-124. | |
| |
Nakada T (1989). On the measurement of cocoon shape by use of image processing method, with an application of sex discrimination of silkworm,Bombyx mori L. Proc. Int. Congr. SABRAO, Japan, 957-960. | |
| |
Nakada T (1992a). The principal component analysis regarding cocoon shape in the silkworm with special reference to the difference between female and male. Abstract, 62nd Congr. Jpn. Soc. Seric. Sci. 92. | |
| |
Nakada T (1992b). Sequence of some cocoon traits in the progeny test after crossing between wild and domesticated silkworms. Wild Silkmoth 17:98-104. | |
| |
Nakada T (1994). On the cocoon shape measurement and its statistical analysis in the silkworm, Bombyx mori L. Indian J. Seric. 33(1):100-102. | |
| |
Nakada T, Maeda H, Murakami M (1991). Discriminant analysis related to sex difference of cocoon shape in the silkworm, Bombyx mori L. Proc. Inst. Statist. Math. 39(2):169-186. | |
| |
Prasad S, Upadhyay VB (2011). Biotechnological importance of cocoon magnetization with particular reference to the larval performance of multivoltine mulberry silkworm (Bombyx mori Linn.). Middle-East J. Sci. Res. 10(5):565-572. | |
| |
Rangaswami G, Narasimhanna MN, Kashivisvanasthan K, Sastry CR (1987). Sericulture manual, 4:21. | |
| |
Rao RMP, Nakada T (1998). Clustering of polyvoltine strains of the silkworm, Bombyx mori by image processing method: significance of cocoon size and weight variables. Indian J. Seric. 37(1):33-39. | |
| |
Sakurai S, Okuda M, Ohtaki T (1986). Juvenile hormone inhibits ecdysone secretion and responsiveness to prothracicotropic hormone in prothrocic gland of Bombyx mori. Gen. Comp. Endocrinol. 75:220-230. | |
| |
Srivastava R Kanchan, Upadhyay VB (2012a). Influence of Phytoecdysteroid on Weight of Ovary and Weight of Multivoltine Mulberry Silkworm (Bombyx mori Linn.). European J. Appl. Sci. 4(3):117-122. | |
| |
Srivastava R Kanchan, Upadhyay VB (2012b). Effect of phytoecdysteroid on length of silk filament and non-breakable filament length of multivoltine mulberry silkworm Bombyx mori Linn. Acad. J. Entomol. 5(3):174-181. | |
| |
Srivastava R, Upadhyay VB (2013a). Effect of bioactive phytojuvenoid on the reproductive potential of multivoltine mulberry silkworm Bombyx mori Linn. (Lepidoptera: Bombycidae). Acad. J. Entomol. 6(2):85-92. | |
| |
Srivastava R, Upadhyay VB (2013b). Influence of Bioactive Phytojuvenoidon the Reproductive Potential of Multivoltine Mulberry Silkworm Bombyx mori Linn. (Lepidoptera: Bombycidae). Acad. J. Entomol. 6(3):139-145. | |
| |
Srivastava R, Upadhyay VB (2013c). Influence of Bioactive Phytojuvenoid on the silk producing Potential of Multivoltine Mulberry Silkworm (Bombyx mori Linn.). IJFBS 1(2):26-31. | |
| |
Sujatha K, Rao PA (2002a). Impact of certain phytochemical substance on the post cocoon characters of Bombyx mori L In: Abstract of National Seminar on mulberry Sericulture Research in India, KSSRDI, Thalaghattapura, Banglore, India, (135):26-28. | |
| |
Sujatha K, Rao PA (2002b). Reserpine (A phytochemical) modulated enhancement of silk productivity in silkworm, Bombyx mori L J. Experimental Zool. India 5(1):57-60. | |
| |
Trivedy K, Nair KS, Assan NM, data RK (1997). A Juvenile hormone mimic modulated enhancement of silk productivity in silkworm, Bombyx mori Ind. J. Serc. 360:35-38. | |
| |
Upadhyay VB, Prasad S (2010b). Biotechnological importance of cocoon magnetization with particular reference to the reproductive potential of multivoltine mulberry silkworm (Bombyx mori Linn). Sericologia, 50(4):461-472. | |