Patterns and trends of macrobenthic abundance, biomass and production in the deep Arctic Ocean


Published: Aug. 26, 2015
Latest article update: Aug. 20, 2023
Abstract
Little is known about the distribution and dynamics of macrobenthic communities of the deep Arctic Ocean. The few previous studies report low standing stocks and confirm a gradient with declining biomass from the slopes down to the basins, as commonly reported for deep-sea benthos. In this study, we investigated regional differences of faunal abundance and biomass, and made for the first time ever estimates of deep Arctic community production by using a multi-parameter artificial neural network model. The underlying data set combines data from recent field studies with published and unpublished data from the past 20 years, to analyse the influence of water depth, geographical latitude and sea-ice concentration on Arctic benthic communities. We were able to confirm the previously described negative relationship of macrofauna standing stock with water depth in the Arctic deep sea, while also detecting substantial regional differences. Furthermore, abundance, biomass and production decreased significantly with increasing sea-ice extent (towards higher latitudes) down to values <200 ind m−2, <65 mg C m−2 and <73 mg C m−2 y−1, respectively. In contrast, stations under the seasonal ice zone regime showed much higher standing stock and production (up to 2500 mg C m−2 y−1), even at depths down to 3700 m. We conclude that particle flux is the key factor structuring benthic communities in the deep Arctic Ocean as it explains both the low values in the ice-covered Arctic basins and the higher values in the seasonal ice zone.
Keywords
Carbon flux, macroinvertebrate, Deep sea, benthos
The density and biomass of marine benthic macrofauna generally decreases with increasing water depth, distance from land, and decreasing latitude from polar and temperate towards tropical latitudes (Gage & Tyler 1991; Levin & Gooday 2003; Wei et al. 2010). The driving force behind this pattern is the decrease in food input, depending on the regionally varying surface production and the assimilation efficiency in the water column (Gage & Tyler 1991; Levin & Gooday 2003 and references therein). The low food concentration in the deep sea leads to a higher share of smaller organisms in total community metabolism—Thiel's (1975) size structure hypothesis. This observation has been corroborated by more recent studies that found a decrease in mean body mass (M) or size with increasing water depth (McClain et al. 2006; Rex et al. 2006; Wei et al. 2010). Besides food availability, substrate characteristics and hydrodynamic processes are also important factors structuring benthic communities
(Rosenberg 1995). Deposit-feeding organisms are reported to dominate areas of reduced flow like the abyssal plains, while suspension feeders are prominent in areas with high bottom current flow, as on continental slopes or mid-ocean ridges (Gage & Tyler 1991; Thistle 2003). Compared to standing stock, little is known about patterns of benthic secondary production (?) and productivity in the deep sea. The production to biomass (P/В) ratio represents the rate of biomass turnover and is inversely related to life span (Benke 2012). A population whose size structure is dominated by small, fast-growing organisms will show a higher P/В ratio than one consisting of older and slower growing adults (Gage & Tyler 1991). P corresponds to the newly formed biomass per unit of area and time and depicts—contrary to pure measurements of biomass—exactly that quantity of energy that is available as food for the next trophic level (Brey 2001). Thus P constitutes the quantitative base of energy flow in benthic food webs and is as such an essential variable for ecosystem models. The few existing studies of deep- sea benthic P report a negative correlation with water depth and low values of 0.1-0.2 g C m-2 y-1 at depths below 1500 m (Gage 1991; Brey & Gerdes 1998; Cusson & Bourget 2005). Benthic community P/В ratios of 0.49y-1 (Gage 1991) and0.55 y-1 (Brey & Gerdes 1998) are reported from 2900 m depth in the Rockall Trough (North Atlantic) and in the Weddell Sea. While two studies detected a negative correlation of P/В ratios with water depth (Brey & Clarke 1993; Cusson & Bourget 2005), no significant correlation was found by Brey & Gerdes in 1998. All the previously mentioned studies detected a positive relation of P/В with temperature. Today, information about Arctic deep-sea benthic communities is even scarcer than information about these communities in the deep sea more generally. This is due to the logistical challenges of sampling the remote, seasonally or permanently ice-covered Arctic basins. Bluhm et al. (2011) found a significant negative correlation of macrobenthic abundance and biomass with water depth and latitude. Based on a thorough literature review, they characterized the Arctic deep sea as an oligotrophic area with steep gradients in faunal abundance and biomass from the slopes to the basins, but with overall density and biomass comparable to other deep-sea areas. Because of permanent ice cover in the central Arctic, surface productivity and associated fluxes are low and previous studies detected extremely low abundances: <200 individuals m-2 and biomasses, <0.2 g carbon (C) m-2 (Klages et al. 2004; MacDonald et al. 2010; Bluhm et al. 2011). Nevertheless, comparably low values of 100 individuals m-2 and 0.5 g wet biomass m-2 have been reported from deep-sea regions equally characterized by remoteness from land and low surface productivity, namely the central North Pacific, the Sargasso Sea and the Porcupine Abyssal Plain (Gage & Tyler 1991).
The recent substantial decrease in the ice cover of the Arctic Ocean (Arrigo et al. 2008) has fuelled speculation as to the future of its productivity and related changes in community structure and distribution. The shift from an Arctic Ocean whose centre is the covered with a thick layer of multiyear ice, and surrounded by a seasonal ice zone, to a system with a mostly seasonal ice zone is already happening (Notz 2009). Arctic marine ecosystems are expected to change accordingly (Wassmann et al. 2011). Currently, neither the direction nor mode of these ecological developments is understood sufficiently to predict forthcoming changes in Arctic marine ecosystem functions, goods and services. One major obstacle is our lack of knowledge regarding the current system state, as quite often there is no reliable baseline information from which change can be identified (Wassmann et al. 2011). As the changes in sea-ice cover and surface productivity are ongoing, it is highly important to increase efforts in establishing such baseline information, including the synthesis of previously unpublished data. Here, we focus on the Arctic deep-sea macrozoobenthos. Deepwater benthic communities are believed to be good indicators of change as they are on average more stationary and long-lived compared to pelagic communities and rely nutritionally almost entirely on the organic flux from euphotic layers. Hence they reflect changes in surface layer production in their own dynamics (Sibuet et al. 1989; Gage & Tyler 1991).
We compiled data on macrozoobenthic communities sampled during expeditions of the RV Polarstem between 1990 and 1997 and in 2012 to the deep Pram Strait and the central Arctic (Fig. 1, Table 1) and estimated benthic P/B and P by applying the empirical artificial neural network model developed by Brey (2012). Based on this data set, we tested patterns previously reported (i.e., decrease of standing crop with depth and latitude, decrease of M with depth, distribution patterns of feeding types), and investigated additional drivers of macrozoobenthic community patterns. In order to identify the major spatial patterns in the data set, we grouped the sample stations into regional and latitudinal clusters, depth zones and zones of different sea-ice concentration and tested these groups for significant differences in their communities' abundance, biomass, M, P, P/В and feeding structure. There are a few estimates of total macrobenthic P from deep-sea regions (Gage 1991; Brey & Gerdes 1998) and high latitudes (Nilsen et al. 2006; Kedra et al. 2013), but none are available yet from the central Arctic deep sea. Our first estimates of benthic P in the Arctic deep sea can serve as an initial baseline for comparisons on a regional and basin-wide scale to help understand and predict upcoming changes in the Arctic Ocean.
Briefly, the main hypotheses tested were: (1) macrobenthic abundance, biomass and P decrease with increasing water depth, latitude and sea-ice coverage; (2) community P/В increases with depth as a consequence of M decreasing with depth; (3) deposit-feeding organisms dominate in the basins, whereas feedings structures are more evenly distributed on the slopes and ridges.
Methods
Study area and data set
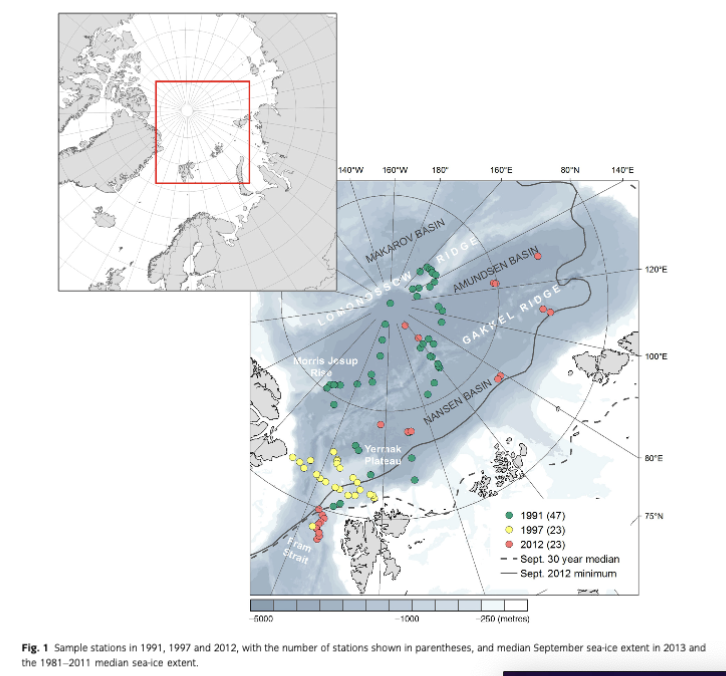
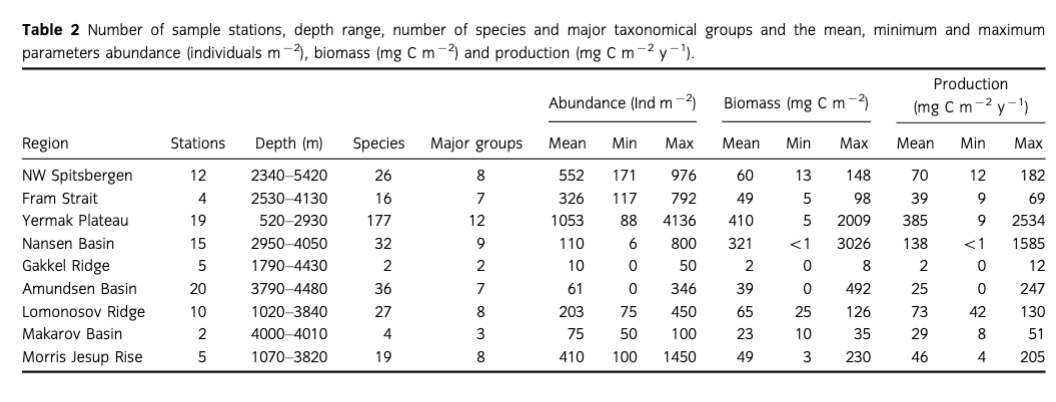
The study area ranges from the seasonally ice-covered eastern Pram Strait (78°N) up to the permanently ice- covered central Arctic Ocean at 90°N. In the region of north-western Spitsbergen and Pram Strait, water depths down to 5600 m are reached at its deepest site, the Molloy Hole (Soltwedel et al. 2005). The inflow of warm Atlantic water that enters the Arctic Ocean via the West Spitsbergen Current keeps the southern stations only seasonally ice-covered. Eastward the West Spitsbergen Current splits up into the Svalbard Branch and the Yermak Branch, both affecting the sea-ice conditions on the Yermak Plateau. This shallow, marginal plateau, located between 80 and 82 °N, north-west of Spitsbergen, ranges from 500 to 800 m on the crest down to 3000 m as it merges into the Nansen Basin (Soltwedel et al.) . Northwards the Nansen and Amundsen basins adjoin, with average depths of 4000 m and most areas permanently covered with sea ice. The two basins are separated by the Gakkel Ridge, a slowly spreading ridge system rising up to 1000 m below sea level (Jakobsson et al. 2012). The Amundsen Basin is limited by the Lomonosov Ridge, which rises 3000 m above the abyssal plains and separates the Eurasian and Amerasian basins (Kristoffersen et al. 2007). The Makarov Basin, flanking the Lomonosov Ridge from the opposite side, is the only region from the Amerasian part of the Arctic included in this study. The western Amundsen Basin merges into the steep slopes of the Morris Jesup Rise, which reaches up to 1000 m below sea level and then transitions into the Greenland Slope (Jakobsson et al. 2012; for detailed station information see Table 2 and Supplementary Table SI).
The data set used for this study (www.doi.pangaea.de/10.1594/PANGAEA.828348; Fig. 1) constitutes a compilation of Alfred Wegener Institute's Arctic macrozoobenthos data (PANABIO, Pan-Arctic Database of Benthic Biota, in progress) selected using the following criteria: (1) abundance and biomass data available on species level; (2) comparable sampling and sample treatment (comparable sampling device and sample area and sieving of samples with 250 pm or 500 pm sieve sizes) to keep comparison errors to a minimum; and (3) data distributed along a transect from the Fram Strait (78°N) to the central Arctic (90°N), with a focus on the Eurasian basins. The samples were taken during several RV Polarstem cruises between 1991 and 2012 (Table 1). Data from the cruise ARK- VHI/3 in 1991 (Fiitterer 1992; 47 stations from northern Svalbard, Yermak Plateau, Morris Jesup Rise and Arctic ridges and basins) were published by Kroncke (1994, 1998) and samples from cruise ARK-XXVII/2 (11 stations from the long-time deep-sea observatory Hausgarten, herein referred to as a group as NW Spitsbergen) by Soltwedel 2013. Data from ARK-XXVII/ 3 in 2012 (Boetius 2013; five stations in Nansen Basin and seven in Amundsen Basin) as well as samples from the cruise ARK-XIII/2 in 1997 (Stein & Fahl 1997; 23 stations: Yermak Plateau, Fram Strait) are provided here (Table 1, Fig. 1).
Sampling procedure
USNEL-type box corers of 0.25 m2 surface area (Gage & Bett 2005) were used for sampling benthic macrofauna on ARK-Vni/3 (see Kroncke 1994, 1998), ARK-XIII/2 and ARK-XXVII/2. Up to seven subsamples of 0.02 m2 were taken per box core on ARK-VIII/3 and ARK-XIII/2. The total surface of a box core was sampled in ARK-XXVII/2 (Soltwedel 2013). On ARK-XXVII/3 samples were taken with a multigrab (9 x 0.024 m2) and benthic chambers of a bottom lander system (3 x 0.04 m2). Single chambers from lander and multigrab deployments were treated like replicate subsamples of box corers from other cruises. The samples from ARK-VIII/3 and ARK-XXVII/2 were washed over 500 pm sieves (top 14 cm), the samples from ARK- XIII/2 with 250 pm sieves (top 2 cm) and 500 pm sieves (2 to max. 20 cm) and samples from ARK-XXVII/3 only with 250 pm sieves (top 10 cm). All samples were stored in 4% (at ARK-XXVII/2 10%) borax-buffered formalin.
In laboratories, macroinvertebrates were counted, weighed (wet weight) and identified to the lowest possible taxonomic level. Generally, all metazoan animals retained on a sieve with 250 or 500 pm mesh size were included in the analysis; only significantly larger animals belonging to the size class "megafauna" ( > 2 cm) were excluded. We are aware that estimates of macrofauna distribution are affected by gear design, sampling area, sample depth and sieve mesh sizes (Wei et al. 2010). Abundance estimates seem more affected by differing sieve mesh sizes than biomass estimates (Shirayama & Horikoshi 1989; Romero- Wetzel & Gerlach 1991; Gage et al. 2002). Gage et al. (2002) showed that 95% of the biomass retained on a sieve with 250 pm mesh size could still be retained on a much coarser sieve of 1 mm mesh size, while about 40% of abundance would be lost when switching from a 250 pm sieve to a sieve with only 500 pm mesh size. Because of this effect, we have to consider an underestimation of abundances by 500 pm samples. Sample area and depth of sample horizon are thought to have comparatively less impact on both abundance and biomass (Gage et al. 2002; Hammerstrom et al. 2012). To exclude potential effects of sampling procedure on our results we performed a three-way ANOVA of the factors sieve size, sample area and year of sampling on the residuals of an ANOVA of abundance (p =0.97) and biomass (p = 0.80) versus regions. The ARK-VIII/3 data set was provided as the median of all subsamples per station (Kroncke 1994, 1998) while the remaining data set consists of mean values per station. No significant "median/mean effect" was detected by an a priori pairwise test mean versus median across all ARK-XXVII/3 stations sampled with the bottom lander system (p =0.708).
Data harmonization
All geographical coordinates were converted to decimal degree. The station data were plotted on a modified polar stereographic International Bathymetric Chart of the Arctic Ocean base map (www.ibcao.org; Jakobsson et al. 2012) in the WGS84 coordinate system using ESRI ArcGIS 10.1.
The taxonomic name of each species was matched with the World Register of Marine Species (WoRMS) as the first authority and also with the Integrated Taxonomic Information Service for reasons of comparability with other data sets. When abundance and biomass data were not already provided per m2 from the start they were recalculated to individuals and g wet mass per m2. A complete list of species taxonomy, abundance, biomass and P can be found in the PANGAEA open access library (www.doi.pangaea.de/10.1594/PANGAEA.828348).
Environmental data
Water depth refers to the recorded depth at the time the sampling device was deployed at the seafloor; bottom water temperature (°C) data were compiled using the PANGAEA open access library (www.pangaea.de). If temperature was not measured during sampling, we used data from nearby conductivity-temperature-depth stations from the same cruise. If no such data were available, we searched for the spatially and temporally closest measurement available from other cruises. This approach is reasonable as the seasonal variations in bottom water temperature from stations below 800 m depth are negligible (Langehaug & Falck 2012). Information about sea-ice concentration (%) per station was extracted from GeoTiff pictures of sea-ice concentration for the respective year and month (25 km raster cells). Sea-ice maps used for the cruises from 2012 were provided by the Institute of Environmental Physics at the University of Bremen (www.iup.uni-bremen.de). For stations sampled before 2002, the pictures used were provided by the National Snow and Ice Data Center (www.nsidc.org/).
The P/В model
Estimation of benthic P was performed using the empirical artificial neural network model developed by Brey (2012). The difference and advantage of this model compared to other empirical models based on multiple linear regression is that it can model complex, non-linear and non-continuous relationships between independent and dependent variables by learning and generalizing from example data (Brey 2012). The P/В model used here is based on an initial database of 1258 data sets, each providing information on annual P, biomass, M, annual P/В ratio, taxonomy and ecology per species as well as the applied methods. The final model (which is implemented in an Excel spread sheet and can be accessed at www.thomas-brey.de/science/virtualhandbook/ [Brey) consists of three continuous and 17 categorical input parameters: M (log(M), [J]), temperature (1/T, [K]), water depth (log(D), [m]), five taxonomic categories (Mollusca, Annelida, Crustacea, Insecta, Echinodermata), seven lifestyle categories (infauna, sessile, crawler, facultative swimmer, herbivore, omnivore, carnivore), four environmental categories (lake, river, marine, subtidal) and a marker for exploitation.
All categorical variables were binary (0 or 1). The necessary ecological information for each species was extracted from the literature and online resources (see below and Supplementary file for details). M was calculated for each species by dividing biomass by abundance. Biomass data were previously converted to Joule, using the conversion factor database of Brey (2012, database version 4, www.thomas-brey.de/science/virtualhandbook). When no conversion factor was found for a certain species the conversion factor of the next higher taxonomical level was used. Species that did not belong to any of the five taxonomic categories of the model were grouped by the category their body form most resembled. Accordingly we grouped Porifera, Tunicata, Cnidaria and Bryozoa by category Mollusca, and Sipuncula, Nemertea, Entoprocta and Cephalorhyncha were grouped by category Annelida. The exploitation marker indicates whether a species is commercially exploited and was set to zero for each species in this study. The model output is population P/B ratio (y-1), including upper and lower 95% confidence limits; population P was calculated by multiplying the P/В ratio with population biomass and community P by adding up all population values. For further details about the model, see Brey (2012).
Functional traits
Information about lifestyle, motility and alimentation type needed as input into the P/В model (see above) was obtained from the literature and through internet search engines like WoRMS (www.marinespecies.org), the Marlin Life Information Network (www.marlin.ac.uk) and the Marine Species Identification Portal (www.species- identification.org/). When no information was found for a certain species the next taxonomic level was tried until reliable information was found. A list of sources consulted (mainly for the two most prominent taxonomic groups in this study, the Annelida and Arthropoda) is included in the Supplementary file.
For the analysis of the trophic group structure of macro- zoobenthic communities, the feeding types were assessed from the same sources as above and assigned to one of these four groups: camivore/predator/scavenger; filter and suspension feeder; interface feeder; and deposit feeder (combining surface and subsurface deposit feeders).
GIS
For mapping benthic abundance, biomass and P, ArcGIS Desktop (Release 10, 2011, Environmental Systems Research Institute, CA, Redlands, USA), was used. Shapefiles containing the geo-referenced sea-ice extent from 2013 and a 30-year mean were provided by the National Snow and Ice Data Center (www.nsidc.org/data/; Fetterer et al.
Statistical analyses
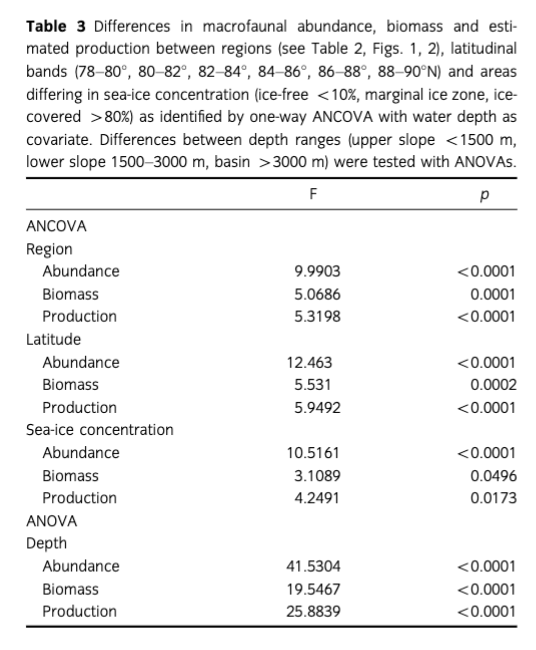
We tested for differences in abundance, biomass, M, P, P/B and feeding structure between (i) regions (NW Spitsbergen, Fram Strait, Yermak Plateau, Nansen Basin, Gakkel Ridge, Amundsen Basin, Lomonosov Ridge, Morris Jesup Rise), (ii) sea-ice zone (i.e., sea-ice concentration in month of sampling: "ice-free"—sea-ice concentration <10%; marginal ice zone [MIZ]—pack ice with concentrations between 10 and 80%; "ice-covered"—sea-ice concentration > 80%), (ill) depth zone (upper slope < 1500 m, lower slope 1500-3000 m, and Basin > 3000 m) and (iv) latitudinal zone (78-80°, 80-82°, 82-84°, 84-86°, 86-88°, 88-90°N). The similarity profile analysis (SIMPROF) approach was used to test if the environmental parameters (water depth, temperature, sea-ice concentration, longitude, latitude) significantly differ between the compared regions and therefore justify the applied regional clustering. As P/B and M are known to be largely influenced by temperature, we tested for a correlation of temperature with P/B and M and also for regional differences in bottom temperature. Statistical approaches included regression, ANOVA, multi-way ANOVA, ANCOVA and post hoc tests (Student's t) using the JMP® software package, version 10.0 (1989-2007, SAS Institute Inc., Cary, NC, USA). Because of the limited number of samples, we performed one-way ANOVAs and ANCOVAs with water depth used as со-variable to test for significant differences between stations (grouped by regions, latitudes and sea-ice concentration) after eliminating the generally acknowledged impact of depth on benthic communities. As depth and temperature со-vary in the Arctic Ocean, we performed an ANOVA on the residuals of a temperature versus depth regression to test for temperature differences among regions. The Makarov Basin region was excluded from the statistical comparison of regions on account of the small sample size of only two stations; all other regions contained 4-20 stations (Table 2). To exclude potentially distorting effects of sampling procedure (i.e., sieve size,
sample area, year of sampling) on our regional comparison we performed a three-way ANOVA of these factors on the residuals of an ANOVA of abundance versus depth and biomass versus depth. Data from regions that were sampled in 1991 and 2012 (Nansen and Amundsen basins) were additionally tested with ANCOVA for an effect of time. Data were transformed using power (Box- Cox) and log transformation. ANOSIM was used to test for differences in the relative contribution of different feeding types to overall biomass and P. SIMPROF and ANOSIM were performed with PRIMER, Version 6 (Clarke & Gorley 2006).
Results
Effects of environment and sampling procedure
The SIMPROF test based on latitude, longitude, temperature and sea-ice concentration found eight significantly different groups (p <0.001) that correspond to the nine regional groups, except for the two Makarov Basin stations, which were grouped together with Lomonosov Ridge stations. Temperature differed significantly between regions (ANOVA with the residuals of a temperature vs. depth regression; F = 2.17; p =0.0449).
The three-way ANOVA of the residuals of an ANOVA of abundance per regions (F = 20.81; p = 0.001) and biomass per regions (F = 9.96; p =0.001) on the factors sieve size, sample area and year of sampling did not find them explaining any variance in abundance (F = 0.25; p =0.97) and biomass data (F = 0.54; p =0.8022). The a priori pairwise test of median versus mean abundances did not detect significant differences for the ARK-XXVII/3 stations (F = 0.15; p =0.708). ANCOVA with depth as a co-variable found abundance and biomass in Nansen Basin significantly higher in 1991 compared to 2012 (F = 11.52; p =0.007 and F = 5.44; p =0.042), but in Amundsen Basin significantly higher in 2012 compared to 1991 (F = 6.58;p =0.021 and F= 11.13; p =0.004).
Abundance
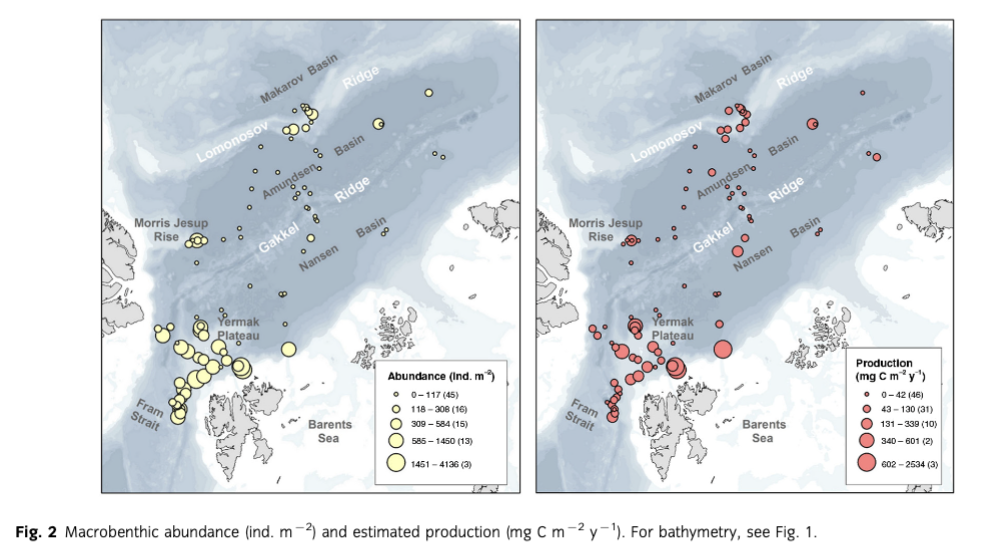
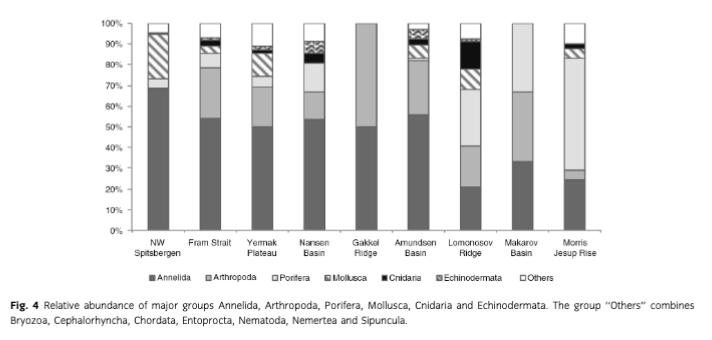
Mean abundance (individuals [ind.] m-2) per region varied between 10 (Gakkel Ridge) and 1053 ind. m-2 (Yermak Plateau) (Table 2, Fig. 2). The highest abundance by far was found at Yermak Plateau at a water depth of 517 m (4136 ind. m-2). Stations from NW Spitsbergen, Morris Jesup Rise and Fram Strait showed relatively high average abundances of 552, 410 and 326 ind. m-2, respectively. All other regions showed lower mean abundances that ranged between 10 and 203 ind. m-2. The lowest abundances were found at the stations in the central Arctic, with means of 90 ind. m-2 and lowest counts of 0 ind. m-2 in Amundsen Basin and at Gakkel Ridge (Table 2). Because water depth was found to have a significant effect on abundances (ANOVA, F =41.53; p< 0.0001; Table 3), it was accordingly used as a covariable in ANCOVAs to test for differences between stations grouped by regions, latitudes and sea-ice concentration (Table 3, Fig. 3). Abundance (ind. m-2) was significantly different between the different regions (F = 9.99; p <0.0001), latitudinal zones (F = 12.46; p <0.0001) and areas of different sea-ice concentration (F = 10.52; p = 0.0005; Fig. 3). The post hoc tests (Student's t) grouped the regions with highest abundance values per m2, i.e., NW Spitsbergen and Yermak Plateau (mean abundance per station 552 and 1053 ind. m2) as significantly different from the regions with stations at greater depths and higher latitudes (i.e., Nansen Basin, Amundsen Basin, Lomonosov Ridge and Morris Jesup Rise, with average abundances between 61 and 410 ind. m-2). The Gakkel Ridge stations were also significantly different from all the other stations as they showed the lowest abundances (zero abundance in four of five stations and one station with 50 ind. m-2). Regarding latitude, abundance was significantly higher at 78-82°N compared to 82-90°N, whereas the stations between 86 and 88°N showed significantly lower values than all the other stations. The northernmost stations, between 88 and 90°N, were ranked third highest, although not significantly different from the stations between 82 and 86°N. When stations were grouped according to percentage of sea-ice concentration with water depth as a co-variable, the stations in the ice-free and MIZ groups did not show significantly different abundances, but were both grouped as significantly different from the ice-covered group (F —10.52; p<0.0001; Table 3, Fig. 3). Of the major taxonomic groups Annelida was by far the most prominent group, ranging from 21% at Lomonosov Ridge up to 68% at NW Spitsbergen (Fig. 4). The second dominant taxonomic group was Arthropoda, with ranges of 25-50% at Gakkel Ridge, Makarov Basin, Amundsen Basin and Fram Strait, but lower contributions in all other regions (1-20%). Porifera was the third most prominent group, with a high share of 27-54% at Lomonosov Ridge, Makarov Basin and Morris Jesup Rise and lower contributions of 0-14% in the other regions. Mollusca had a higher share of the total community, with 22% only at NW Spitsbergen. They grouped with all other phyla (Bryozoa, Cephalorhyncha, Chordata, Cnidaria, Echinoidea, Entoprocta, Nematoda, Nemertea and Sipuncula) in the lower range of 0-14% at other regions (Fig. 4, Supplementary Table S2).
Biomass
Mean biomass per region ranged from 2 mg C m-2 at Gakkel Ridge up to 410 mg C m-2 at Yermak Plateau (Table 2). The highest biomass by far was found at Yermak Plateau and Nansen Basin stations (max. 2009 and 3026 mg C m-2), while all other regions showed low mean biomass, ranging between 2 and 65 mg C m-2. Because water depth was found to have a significant effect on biomass (ANOVA, F —19.55; p < 0.0001, Table 3), it was used as со-variable in the following ANCOVAs (Table 3, Fig. 3). ANCOVAs detected significant differences in biomass between regions (F —5.07; p <0.0001) (Table 3, Fig. 3). Post hoc tests grouped the stations from Yermak Plateau (mean biomass 410 mg C m-2) as significantly different to those of Amundsen Basin, Morris Jesup Rise and Gakkel Ridge. No significant difference was detected to stations from NW Spitsbergen, Fram Strait, Nansen Basin and Lomonosov Ridge. With respect to latitude, a significant difference was found between stations (F —5.53; p =0.0002). Here the stations between 80° and 82 °N were found to be significantly higher in biomass than all the stations of the areas 82-84, 84-86 and 86-88°N, but were not found to be significantly different from the southernmost (78-80°N) and northernmost (88-90°N) stations. Comparing stations by seaice concentration showed significantly higher biomasses for the stations in the MIZ group (F = 3.11; p =0.0496) compared to the ice-covered group. The ice-free group was not significantly different from the other two groups (Table 3, Fig. 3). Annelids contributed most to community biomass at Morris Jesup Rise (86%) and NW Spitsbergen (74%), and between 10 and 60% elsewhere. Arthropoda contributed 58% of the biomass in Amundsen Basin, 40% at Gakkel Ridge and 34% in Fram Strait, but only between 0 and 17% in all other regions. Echinoderms dominated biomass in Nansen Basin (66%), but showed rather low percentages at all other regions (0-12%). Porifera dominated the community biomass in Makarov Basin (60%) and contributed a lot in Pram Strait (45%), at Lomonosov Ridge (32%) and Nansen Basin (21%). Mollusca showed relevant shares of 29% at Lomonosov Ridge; in other regions they contributed <4%. All other groups did not contribute significantly to community biomass and ranged between 0 and 10% in all regions (Fig. 5, Supplementary Table S2). Regarding trophic structure, deposit feeders were the dominant group, while interface feeders had significantly lower biomasses (F = 14.61; p <0.0001). Deposit feeders had a much higher share, with 66% of total biomass at Nansen Basin and 4-60% in the other regions except Makarov Basin. Carnivores/predators/scavengers contributed most at Morris Jesup Rise (84%) and Amundsen Basin (67%). Filter feeders dominated the biomass at Makarov Basin (60%) and Lomonosov Ridge (53%; Supplementary Fig. S2). ANO SIM did not detect differences in the relative contribution of different feeding types between any of the tested groups (depth, latitude, sea ice and region; Global R< 0.20).

Mean body mass (M)
M of the stations from Nansen Basin, Lomonosov Ridge and Yermak Plateau with values between 0.4 and 2.5 mg C were significantly higher than at NW Spitsbergen, Morris Jesup Rise and Gakkel Ridge, with values of 0.03-0.1 mg C (F —3.12; p = 0.0028). While no significant differences in M were found within the different water depths (F —0.73; p =0.4835) and sea-ice zones (F= 1.87; p = 1398), we detected significant differences between latitudinal zones (F = 2.83; p =0.0207). Post hoc tests ranked the groups 86-88°N and 78-80°N to be significantly lower than the groups 88-90°N, 80-82°N and 82-84°N. M was not significantly related to bottom water temperature (F = 0.01; p =0.9144).
Benthic secondary production (P)
Mean macrobenthic P was lowest at Gakkel Ridge with 2 mg C m-2 y-1 and highest at Yermak Plateau with 385 mg C m-2 y-1 (Table 2, Fig. 2). The highest P per station was found at Yermak Plateau (reaching up to 2534 mg C m-2 y-1), followed by Nansen Basin, with values reaching 1585 mg Cm-2y-1.Pat NW Spitsbergen and Lomonosov Ridge was rather similar, with means of 70 and 73 mg Cm”2y-1, respectively. All other regions ranged in their means between 2 and 46 mg Cm"2y-1. Because water depth was found to have also a significant effect on P (ANOVA, F = 2 5.88; p < 0.0001; Table 3), it was used again as a со-variable in ANCOVAs (Table 3, Fig. 3). ANCOVAs showed that there were significant differences between regions (F —5.32; p < 0.0001; Table 3). Post hoc tests grouped the regions with highest mean P (Yermak Plateau, NW Spitsbergen and Lomonosov Ridge) and the stations with lowest mean P (Morris Jesup Rise and Gakkel Ridge) to be significantly different from each other. Also when grouped by latitude, significant differences were found by ANCOVA (F = 5.95;p <0.0001).Post hoc tests revealed that the benthic P from stations at 80—82°N, 78—80°N and 88-90°N was significantly higher than in the groups at 84-88°N. Comparison of stations grouped by their sea-ice concentration also showed significant differences in P (F=4.25;p =0.0173). As for biomass, post hoc tests showed significantly higher benthic P in the MIZ group (mean 0.5 g C m-2 у-1) compared to the Recovered group (mean 0.06 g C m-2 y-1; Table 3, Fig. 3). Annelids contributed most to the overall P at NW Spitsbergen (73%), at Morris Jesup Rise (67%), at Yermak Plateau and Gakkel Ridge (both 64%) and at Amundsen Basin (51%) (Fig. 6, Supplementary Fig. S2). At the other regions they contributed between 14 and 25% to the overall P. Porifera were the most productive group at Makarov Basin (70%), Nansen Basin (49%) and Lomonosov Ridge (47%). Arthropoda contributed45% atFram Strait, 39% at Amundsen Basin and 36% at Gakkel Ridge, but only between 0 and 16% in all other regions. All other groups contributed much less to the overall P. Echinoderms contributed 23% to the overall P at Nansen Basin but only between 0 and 6% in other regions. Molluscs only showed a considerable percentage at Lomonosov Ridge (12%) but ranged at all other stations between 0 and 3%. Suspension feeders had the largest share in P, while deposit feeders showed the significantly lowest values (F = 30.22; p <0.0001). In the three depth zones, suspension feeders contributed most in the lower slope group (50%) and comparably less to the upper slope group (17%) and "basins" (24%). At a regional scale, filter and suspension feeders contributed most to P at Makarov Basin (70%), Nansen Basin (64%) and Lomonosov Ridge (55%), predators at Morris Jesup Rise (65%), deposit feeders at Gakkel Ridge (64%), Amundsen Basin (50%) and Yermak Plateau (48%), and interface feeders at NW Spitsbergen (42%; Fig. 7). ANOSIM did not detect differences in the relative contribution of different feeding types in any of the categories tested (depth, latitude, sea ice, region; Global R always <0.20).
Production to biomass ratio (P/B)
P/В ratios ranged from 0.14 to 2.22 and were highest at Morris Jesup Rise, Lomonosov Ridge and NW Spitsbergen, with means per region ranging from 1.17 to 1.42 y-1. Gakkel Ridge was the region with the significantly (F = 3.13; p =0.0057) lowest P/В ratio (mean = 0.29 у-1). ANOVA and ANCOVA did not detect differences in P/В between depth zones (F = 1.34; p =0.265), latitude zones (F = 1.56;p=0.1690) and zones of different sea-ice concentration (F = 1.15; p =0.3212). Among major taxonomic groups Porifera and Arthropoda had highest mean ratios of 1.28 and 1.25 y-1. Regarding trophic structure, deposit and suspension feeders showed significantly higher ratios than interface feeders and predators (F = 2.96; p =0.03). P/В was significantly positively related to bottom water temperature (F = 10.01; p =0.002), but not to M (F = 3.28; p =0.0733).

Discussion
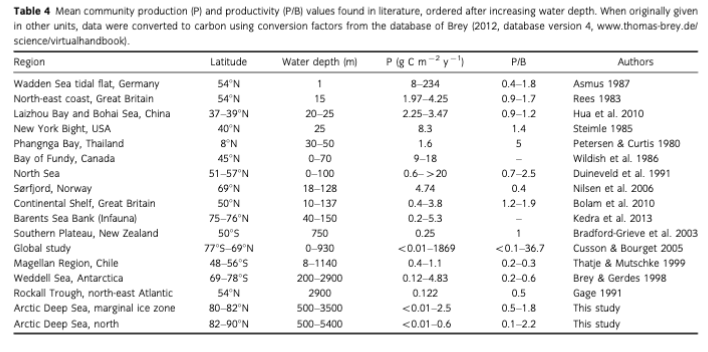
Macrofauna standing stock and P in the Arctic deep-sea decrease with increasing water depth. In addition, we detected significant regional differences for all studied community properties (abundance, biomass, M, P and P/В). Stations in the vicinity of the highly productive MIZ (latitudes 80-82°N) showed P levels comparable to shallower regions and lower latitudes (Table 4). In the permanently ice-covered central Arctic Amundsen Basin, mean macrobenthic P was estimated to be as low as 25 mg C m-2 y-1 (Table 2). Assuming an average production-to-consumption ratio (P/C) of macrofauna of about 0.2 (0.239 + 0.190, N = 97; unpublished data collection of T. Brey), this P would require a particulate organic carbon (РОС) input of at least 165 mg C m-2 y-1 for the macrofaunal consumption only, which is presumably 20% of all benthic size classes including bacteria (Piepenburg et al. 1995). Based on the assumption that <10% of surface primary production reaches the deep-sea floor (Bauerfeind et al. 2009), a gross primary production (GPP) of around 8 g C m-2 у-1 would be sufficient to cover this benthic demand. This number is well in the range of reported GPP estimates of 1-25 g C m-2 y-1 for the central Arctic (Wassmann et al. 2010). Sufficiently high РОС fluxes of >1 g C m-2 y-1 were also recorded via sediment traps situated at 1550 m of depth (Pahl & Noting 2007). In contrast to the central Arctic stations, we estimated a mean P of 385 mg C m-2 y-1 at the Yermak Plateau. Taking into account that at shallower depths (mean 1500 m) a higher percentage of GPP can reach the seafloor, a GPP of approximately 30-90 g C m-2 у-I would be required to enable the estimated community P. In the Arctic, such a high primary productivity can be found regionally along the highly productive seasonal ice zone and in productive shelf areas like in the Barents Sea (Klages et al. 2004; Wassmann et al. 2010), which are both in the vicinity of and most likely affecting our sample stations. We conclude that particle flux induced by vertical and lateral transport processes is the key factor structuring benthic communities in the deep Arctic Ocean, explaining both the very low values in the ice-covered Arctic basins and the higher values in the seasonal ice zone.
Depth-related patterns
Our study confirms the trends shown earlier (Gage & Tyler 1991; Klages et al. 2004; Bluhm et al. 2011): Significantly lower mean abundances and biomasses are found in the deep basins compared to the upper slopes adjacent to the large Arctic shelves (F=41.53; p < 0.0001; respectively F = 19.55; p <0.0001; Table 3, Fig. 2).
Mean abundance at the upper slope below 1500 m water depth ranges between 100 and 4130 Ind. m-2 (Table 2, Fig. 2), consistent with abundances summarized in Bluhm et al. 2011 and Budaeva et al. 2008, and comparable to or even higher than abundances at lower latitudes from previous studies at similar depth ranges (see e.g., Levin & Gooday 2003). Estimated benthic P was shown to follow the same pattern, i.e., significant differences between shallower and deeper stations (F = 25.88; p <0.0001) (Table 2, Fig. 3). This corroborates the pattern of community P decreasing exponentially with water depth, as reported previously by Brey & Gerdes (1998) for a combined data set from Antarctic, Arctic and non-polar regions and by Cusson & Bourget (2005) who analysed global patterns of community P.

Extreme food limitation as found in the deep sea creates selection pressure towards smaller body sizes (Thiel 1975; Wei et al. 2010). Smaller size often coincides with a higher growth rate and thus a higher P/В ratio (Brown et al. 2004). Accordingly, M should decrease and community P/В should increase with increasing water depth (Peters 1983). However, M and P/В ratios did not significantly relate to water depth (F —0.73; p =0.4835 and F = 1.34; p =0.265), in accordance with Polloni et al. (1979) who did not find a decline in mean macrofaunal organism size from 400 to 4000 m. Distinctly larger body size seems to be restricted to very shallow (neritic or coastal) waters. Accordingly, data sets that exclude the upper 500 m like in this study may not show depth effects on M, and models that include shallow depths may overstate the depth effect in the deep sea (Wei et al. 2010). On the other hand, Kaariainen & Bett (2006) found clear evidence of smaller body size in the deep sea when evaluating body size accumulation curves, stressing the need for size structure analysis. While no correlation of P/В ratios and water depth was found here, Cusson & Bourget (2005) found a negative relation between P/В and water depth (and a positive relation with temperature), and presume that certain life history traits may explain patterns in P/В ratios better than environmental variables.
Regional patterns
Here we detected significant regional differences—beyond those caused by water depth—for all studied community properties (abundance, biomass, M, P and P/В). The regions Yermak Plateau and NW Spitsbergen (latter only in abundance) showed significantly higher values than the regions in higher latitudes (i.e., Amundsen Basin and Gakkel Ridge; Table 3, Fig. 3). This pattern is corroborated when stations were grouped by latitude (significantly higher values at 80-82°N and for abundance at 78-80°N) or by ice zone (significantly higher values in the MIZ group; Table 3, Fig. 3). The generally higher values at Yermak Plateau might be explained by its vicinity to the highly productive Barents and Spitsbergen shelves and the high primary production in this region (GPP 30-100 g C m-2 y-1) (Wassmann et al. 2010). The high GPP is supported by Atlantic water supply and the fertile conditions generally found along the MIZ (Sakshaug 2004), which covers a large fraction of northern Fram Strait (Sakshaug 2004; Wassmann et al. 2010). Along ice edges РОС fluxes of >300 mg C m-2 d-1 are
recorded, greatly exceeding those found in open water (intermediate export fluxes 12-27 mg m-2 d-1; Klages et al. 2004). The estimated benthic P in the MIZ group (highest value 2.5 g C m-2 y-1, mean 0.5 g C m-2 y-1; depth of 500-3500 m) is in the lower range of but still comparable to benthic P estimates from the shallow Barents Sea Bank (0.02-5.3 g C m-2 y-1) in depths between 40 and 150 m (Kedra et al. 2013; Table 4), and to shallow areas from temperate regions like the UK Continental Shelf with means ranging from 0.4-3.8 g C m-2 y-1 (Bolam et al. 2010; Table 4). Regarding regional groups, the highest mean P was found at Yermak Plateau with 385 mg C m-2 у-1. The values from the second most productive area (Nansen Basin, mean P of 138 mg C m-2 y-1) from depths between 3000 and 4000 m are—although covered with sea ice throughout most of the year—comparable to values reported from the Rockall Trough in the north-east Atlantic (122 mg C m-2 y-1) in depths of 2900 m (Gage 1991). These comparisons indicate that benthic communities from the Arctic deep sea can be comparable in P to other regions, if they are in the vicinity to the highly productive seasonal ice zone and the continental shelf. The third most productive areas are the southernmost stations in NW Spitsbergen and the northernmost stations on the Lomonosov Ridge (70 and 73 mg C m-2 y-1). While the stations north-west of Spitsbergen benefit from the conditions mentioned previously, the stations in the High Arctic are far from any input from the MIZ and the productive shelf areas. We assume that benthic P at the Lomonosov Ridge could be fuelled by organic matter that gets transported with sea ice along the Transpolar Drift, enhancing export via seasonal melting processes. The stations far off the seasonal ice edge, e.g., in Amundsen or Makarov Basin or on the Gakkel Ridge, show as low P as anticipated for the most oligotrophic deep-sea regions, as primary production under the permanent ice cover is very low (1-25 g C m-2 y-1) (Wassmann et al. 2010). Recent studies have found indications for much higher carbon fluxes associated with sea-ice minima in 2007 (Lalande et al. 2009) and 2012 (Boetius et al. 2013), and the rapid export of sea-ice algae to the seafloor. Our results corroborate these observations, as the significantly higher benthic biomass in the central and eastern Amundsen Basin in 2012 compared to 1991 (F= 11.13; p — 0.004) may indicate an increase in vertical flux over these two decades. However, there are just five samples from 2012 and these were not taken in exactly the same area of Amundsen Basin as in 1991. Hence, this finding should not be over-interpreted; distinctly higher sampling effort is required to produce more reliable data.
Nevertheless, the ongoing decline in sea-ice cover and thickness in the central basins are likely to cause future changes in macrozoobenthos abundance, biomass and P.
While we found no correlations of M and P/В with water depth, we did detect significant regional differences (F = 3.12; p =0.0028; F = 3.13; p =0.0057). Highest P/B ratios were found in the region Morris Jesup Rise, ranging from 0.8 to 2.2 y-1. The most important factors influencing the community P/В ratio are body mass, temperature and food (Brey & Clarke 1993 and references therein). Overall we found no correlation of P/B ratios with M (77 = 3.28; p = 0.0733), but we did detect a positive relation of P/B to temperature (F = 10.01; p =0.002). However, as the temperature difference among regions is small, and the region with the highest P/B values (Morris Jesup Rise) is not the one with the highest temperatures (Lomonosov Ridge), we assume that additional drivers have to be considered. The third proposed explanatory factor, food input, is quite difficult to determine in the ice-covered Arctic Ocean. We presume the highest food fluxes to be in areas influenced by the MIZ and close to shelf regions, i.e., those regions where we found the highest P. But unlike P, P/B ratios where highest in the northern most regions under permanent sea ice (i.e., Morris Jesup Rise and Lomonosov Ridge), where low РОС fluxes of >1 g C m-2 y-1 were measured (Pahl & Nothig 2007). To summarize, although we found a correlation of P/B with temperature, none of the usual drivers of P/B (M, temperature and food input) could satisfyingly explain the observed regional pattern. This may partially be due to the high degree of intercorrelation between temperature, depth, and food input in the Arctic deep sea hampering statistical analysis.
Patterns in feeding structure
Structure and function of benthic communities can be analysed beyond the assessment of basic community parameters, by dividing organisms in groups with shared behavioural traits or with shared resource bases (Cochrane et al. 2012). Here we analysed feeding mechanisms, as they are one of the central determinants of marine ecosystem structure (Bremner et al. 2003), and information can be found in literature or be inferred from feeding or mouth structures (Supplementary file). Cusson & Bourget (2005) found highest P for suspension feeders and highest P/B ratios for omnivores and predators. They explain this result by the fact that this feeding guild is dominated by annelids and arthropods with short life spans, small body mass and high mobility, all factors assumed to enhance the metabolic rate and as such also P/В ratios. The effect of mobility on P/В ratios is controversial though. On the one hand, motile species potentially use more energy for respiration than for growth, leading to lower P/В ratios. On the other hand, mobility enables access to higher quality food, which might lead to higher P/В ratios. This is an important factor especially in the Arctic deep sea, where food falls in the form of carcasses or ice-algae deposits (Boetius et al. 2013) form an important source of nutrition. However, we found suspension feeders to contribute most to overall P (F — 30.22,- p <0.0001), while deposit and suspension feeders displayed higher P/В ratios than interface feeders and predators (F = 2.96; p =0.03). This result might be explained by the fact that highly mobile predators and scavengers are underrepresented in our study, as in the deep sea this group is predominantly represented in the megafauna size class (Gage & Tyler 1991).
Physical dynamics play an important role in determining trophic community structure, with fauna shifting to suspension feeders in hydrographically dynamic areas and deposit feeders in depositional areas (Rosenberg 1995). Accordingly, deep-sea areas with reduced flow and with scarce and low quality food input, such as abyssal plains, are dominated by deposit feeders, while suspension feeders are abundant in areas with high bottom current flow, as on continental slopes and midocean ridges (Gage & Tyler 1991; Thistle 2003). Our findings confirm this general view: deposit feeders contribute most to overall P in the Amundsen Basin (50%), and suspension feeders at the Lomonosov Ridge (55%). However, other regions show a less clear pattern. In the Nansen Basin, suspension feeders contributed 64% and deposit feeders only 24% to overall P. The highest P in the Nansen Basin was found at the stations on the lower Barents Sea and Yermak Plateau slope (Fig. 2), presumably benefitting from bottom current flows and food advection from the Barents Sea shelf. Generally, when stations were grouped into three depth zones (upper slope, lower slope, basin), the highest contribution of suspension feeders was found in the lower slope group (50%). The region with highest P—Yermak Plateau— shows a more even distribution of feeding types than the low productivity regions—Gakkel Ridge and Amundsen Basin (Fig. 7). This indicates a complex benthic food web well adapted to handle the high РОС input found along the MIZ in the vicinity of the productive continental shelf. Although some patterns are apparent, the ANO SIM analysis failed to detect significant differences in the relative contribution of different feeding types between regions, depth zones, latitudinal zones and areas of different sea-ice concentration. Bremner et al. (2003) could show that the biological trait analysis better illuminates the ecological functions of benthic communities than taxonomical or trophic group approaches. Accordingly the biological trait analysis might be a more suitable approach here, but our knowledge about behavioural and life history traits of deep-sea taxa is still limited.
Outlook
This study is a first step in providing baseline data concerning macrobenthic community parameters in the Arctic deep sea based on a data synthesis covering the years 1990-2012 and different regions of the Arctic deep- sea slopes and basins. A major limitation to assessing status changes in the Arctic deep-sea ecosystem remains the poor spatial and temporal resolution of sampling. In light of the observed climatic changes and the rapid decrease of sea-ice volume and cover, it is now important to collect more data at higher spatial resolution. Furthermore, quality control procedures, such as standardized study design (i.e., sample size, sample depth and sieve mesh size), should be implemented. We support the recommendations already stated in previous large-scale studies of the deep-sea macrozoobenthos (e.g., Bluhm et al. 2011) to apply consistent sampling sizes and to use sieves with 250 pm mesh size as a standard, to account for the small body sizes of deep-sea taxa. We further want to stress the importance of geo-referenced data archives and international efforts to synthesize available data, to improve our understanding of current and future changes in the Arctic Ocean ecosystem.
Acknowledgements
The authors thank the captains, crews and shipboard parties of the RV Polarstern ARK-VIII, ARK- ХШ/2, ARK- XXVH/2 and ARK-XXVH/3 cruises for help with work at sea. The authors thank Jblund Asseng (Alfred Wegener Institute) for information and help in importing sea-ice concentration data into Arc GIS and Professor Dr Ursula Schauer (Alfred Wegener Institute) for helpful information about Arctic seawater temperatures. The authors further thank Sergey Y. Gagaev, Alexey V. Golikov and Victor Petrjashev from the Zoological Institute of the Russian Academy of Sciences for the taxonomical work on ARK-Xin/2 samples. The authors also thank the anonymous reviewers for their constructive comments. This work was supported by the European Research Council ABYSS advanced grant (294757) to AB. RD and ТВ were supported by the POLMAR graduate programme.
References
- Arrigo K.R., van Dijken G. & Pabi S. 2008. Impact of a shrinking Arctic ice cover on marine primary production. Geophysical Research Letters 35, L19603, doi: 10.1029/2008 GL035028.
- Asmus H. 1987. Secondary production of an intertidal mussel bed community related to storage and turnover compartments. Marine Ecology Progress Series 39, 251-266.
- Bauerfeind E., Nothig E., Beszczynska A., Fahl K., Kaleschke, Kreker K., Klages M., Soltwedel T., Lorenzen C. & Wegner J. 2009. Particle sedimentation patterns in the eastern Pram Strait during 2000-2005: results from the Arctic long-term observatory HAUSGARTEN. Deep-Sea Research Part 156, 1471-1487.
- Benke A. 2012. Secondary production. Nature Education Knowledge 3, 23.
- Bluhm B.A., Ambrose WG. Jr., Bergmann M., Clough L.M., Gebruk A.V., Hasemann C., Iken K., Klages M., MacDonald I.R., Renaud P.E., Schewe I., Soltwedel T. & Wlodarska- Kowalczuk M. 2011. Diversity of the Arctic deep-sea benthos. Marine Biodiversity 41, 87-107.
- Boetius A. 2013. The expedition of the research vessel "Polarstem" to the Arctic in 2012 (ARK-XXV11/3). Berichte zur Polar- und Meeresforschung 663. Bremerhaven: Alfred Wegener Institute for Polar and Marine Research.
- Boetius A., Albrecht S., Bakker K., Bienhold C., Belden J., Fernandez Mendez M., Hendricks S., Katlein C., Lalande C., Krumpen T., Nicolaus M., Peeken I., Rabe B., Rogacheva A., Rybakova E., Somavilla Cabrillo R., Wenzhofer F. & RV Polarstem ARK27-3-Shipboard Science Party 2013. Export of algal biomass from the melting Arctic sea ice. Science 339, 1430-1432.
- Bolam S.G., Barrio-Frojan C.R.S. & Eggleton J.D. 2010. Macrofaunal production along the UK continental shelf. Journal of Sea Research 64, 166-179.
- Bradford-Grieve J.M., Probert P.K., Nodder S.D., Thompson D., Hall J., Hanchet S., Boyd P., Zeldis J., Baker A.N., Best H.A., Broekhuizen N., Childerhouse S., Clark M., Hadfield M. , Safi K. & Wilkinson I. 2003. Pilot trophic model for Subantarctic water over the Southern Plateau, New Zealand: a low biomass, high transfer efficiency system. Journal of Experimental Marine Biology and Ecology 289, 223-262.
- Bremner J., Rogers S.I. & Frid C.L.J. 2003. Assessing functional diversity in marine benthic ecosystems: a comparison of approaches. Marine Ecology Progress Series 254, 11-25.
- Brey T. 2001. Population dynamics in benthic invertebrates. A virtual handbook. Version 01.2. Accessed on the internet at http://www.thomas-brey.de/science/virtualhandbook/ on 07 2013.
- Brey T. 2012. A multi-parameter artificial neural network model to estimate macrobenthic invertebrate productivity and production. Limnology and Oceanography—Methods 10, 581-589.
- Brey T. & Clarke A. 1993. Population dynamics of marine benthic invertebrates in Antarctic and Subantarctic environments: are there unique adaptations? Antarctic Science 5, 253-266.
- Brey T. & Gerdes D. 1998. High Antarctic macrobenthic community production. Journal of Experimental Marine Biology and Ecology 231, 191-200.
- Brown J.H., Gillooly J.F., Allen A.P., Savage V.M. & West G.B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771-1789.
- Budaeva N., Mokievsky V., Soltwedel T. & Gebruk A. 2008. Horizontal distribution patterns in Arctic macrobenthic deep- sea communities. Deep-Sea Research Part 155, 1167-1178.
- Clarke K.R. & Gorley R.N. 2006. PRIMER v6: user manual/ tutorial. Plymouth, UK: PRIMER-E.
- Cochrane S.K.J., Pearson Т.Н., Greenacre M., Costelloe J., Ellingsen I.H., Dahle S. & Gulliksen B. 2012. Benthic fauna and functional traits along a Polar Front transect in the Barents Sea—advancing tools for ecosystem-scale assessments. Journal of Marine Systems 94, 204-217.
- Cusson M. & Bourget E. 2005. Global patterns of macroinvertebrate production in marine benthic habitats. Marine Ecology Progress Series 297, 1-14.
- Duineveld G.C.A., Kiinitzer A., Niermann U., De Wilde P.A.W.J. & Gray J.S. 1991. The macrobenthos of the North Sea. Netherlands Journal of Sea Research 28, 53-65.
- Fahl K. & Nothig K. 2007. Lithogenic and biogenic particle fluxes on the Lomonosov Ridge (central Arctic Ocean) and their relevance for sediment accumulation: vertical vs. lateral transport. Deep-Sea Research Part 154, 1256-1272.
- Fetterer F., Knowles K., Meier W. & Savoie M. 2002. Sea ice index. Digital media. Boulder, CO: National Snow and Ice Data Center.
- Ftitterer D. 1992. ARCTIC'91: die Expedition ARK-VII1/3 mit FS "Polarstem’ 1991. (ARCTIC’91: the expedition ARK-VHI/3 of RV “Polarstem" in 1991.) Berichte zur Polarforschung 107. Bremerhaven: Alfred Wegener Institute for Polar and Marine Research.
- Gage J.D. 1991. Biological rates in the deep sea: a perspective from studies on processes in the benthic boundary layer. Reviews in Aquatic Sciences 5, 49-100.
- Gage J.D. & Bett B.J. 2005. Deep-sea benthic sampling. In A. Eleftheriou & A. McIntyre (eds.): Methods for the study of marine benthos. Pp. 273-325. Oxford: Blackwell Science.
- Gage J.D., Hughes D.J. & Gonzalex Vedno J.L. 2002. Sieve size influence on estimating biomass, abundance and diversity in samples of deep-sea macrobenthos. Marine Ecology Progress Series 225, 97-107.
- Gage J.D. & Tyler P.A. 1991. Deep-sea biology: a natural history of organisms at the deep-sea floor. London: Cambridge University Press.
- Hammerstrom K.K., Ranasinghe J.A., Weisberg S.B., Oliver J.S., Fairey W.R., Slattery P.N. & Oakden J.M. 2012. Effect of sample area and sieve size on benthic macrofaunal community condition assessments in California enclosed bays and estuaries. Integrated Environmental Assessmental Management 8, 649-658.
- Hua E., Zhou H., Zhang Z. & Yu Z. 2010. Estimates of autumntime benthic secondary production in Laizhou Bay and adjacent Bohai Sea waters. Journal of Ocean University of China 9, 279-285.
- Jakobsson M., Mayer L.A., Coakley B., Dowdeswell J.A., Forbes S., Fridman B., Hodnesdal H., Noormets R., Pedersen R., Rebesco M., Schenke H.-W, Zarayskaya Y., Accettella D., Armstrong A., Anderson R.M., Bienhoff P., Camerlenghi A., Church I., Edwards M., Gardner J.V., Hall J.K., Hell B., Hestvik O.B., Kristoffersen Y., Marcussen C., Mohammad R., Mosher D., Nghiem S.V., Pedrosa M.T., Travaglini P.G. & Weatherall P. 2012. The International Bathymetric Chart of the Arctic Ocean (IBCAO) version 3.0. Geophysical Research Letter 39, article no. L12609, doi: 10.1029/2012GL052219.
- Kaariainen J.I. & Bett B.J. 2006. Evidence for benthic body size miniaturization in the deep sea. Journal of the Marine Biological Association of the United Kingdom 86, 1339-1345.
- Kedra M., Renaud P.E., Andrade H., Goszczko I. & Ambrose WG. Jr. 2013. Benthic community structure, diversity, and productivity in the shallow Barents Sea bank (Svalbard Bank). Marine Biology 160, 805-819.
- Klages M., Boetius A., Christensen J.P., Deubel H., Piepenburg D., Schewe I. & Soltwedel T. 2004. The benthos of Arctic seas and its role for the organic carbon cycle at the seafloor. In R. Stein & R.W. Macdonald (eds.): The organic carbon cycle of the Arctic Ocean. Pp 139-167. Heidelberg: Springer.
- Kristoffersen Y., Coakley B.J., Hall J.K. & Edwards M. 2007. Mass wasting in the submarine Lomonosov Ridge, central Arctic Ocean. Marine Geology 243, 132-142.
- Kroncke I. 1994. Macrobenthos composition, abundance and biomass in the Arctic Ocean along a transect between Svalbard and the Makarov Basin. Polar Biology 14, 519-529.
- Kroncke I. 1998. Macrofauna communities in the Amudsen Basin, at the Morris Jesup Rise and at the Yermak Plateau (Eurasian Arctic Ocean). Polar Biology 19, 383-392.
- Lalande C., Belanger S. & Fortier L. 2009. Impact of a decreasing sea ice cover on the vertical export of particulate organic carbon in the northern Laptev Sea, Siberian Arctic Ocean. Geophysical Research Letters 36, L21604, doi: 10.1029/ 2009GL040570.
- Langehaug H.R. & Falck E. 2012. Changes in the properties and distribution of the intermediate and deep waters in the Fram Strait. Progress in Oceanography 96, 57-76.
- Levin L.A. & Gooday A.J. 2003. The deep Atlantic ocean floor. In P.A. Tyler (ed.): Ecosystems of the deep oceans. Pp. 111-178. Amsterdam: Elsevier Science.
- MacDonald I.R., Bluhm B.A., Iken K., Gagaev S. & Strong S. 2010. Benthic macrofauna and megafauna assemblages in the Arctic deep-sea Canada Basin. Deep-Sea Research Part II 57, 136-152.
- McClain C.R., Boyer A.G. & Rosenberg G. 2006. The island rule and the evolution of body size in the deep sea. Journal of Biogeography 33, 1578-1584.
- Nilsen M., Pedersen T. & Nilssen E.M. 2006. Macrobenthic biomass, productivity (P/В) and production in a high- latitude ecosystem, north Norway. Marine Ecology Progress Series 321, 67-77.
- Notz D. 2009. The future of ice sheets and sea ice: between reversible retreat and unstoppable loss. Proceedings of the National Academy of Sciences of the United States of America 106, 20590-20595.
- Peters R.H. 1983. The ecological implications of body size. Cambridge: Cambridge University Press.
- Petersen G.H. & Curtis M.A. 1980. Differences in energy flow through major components of Subarctic, temperate and tropical marine shelf ecosystems. Dana 1, 53-64.
- Piepenburg D., Blackbum TH., von Dorrien C.F., Gutt J., Hall P.O.J., Hulth S., Kendall M.A., Opalinski K.W., Rachor E. & Schmid M. 1995. Partitioning of benthic community respiration in the Arctic (northwestern Barents Sea). Marine Ecology Progress Series 118, 199-213.
- Polloni P., Haedrich R., Rowe G. & Clifford C.H. 1979. The size-depth relationship in deep ocean animals. Internationale Revue der gesamten Hydrobiologie und Hydrographie 64, 39-46.
- Rees H.L. 1983. Pollution investigations off the north-east coast of England: community structure, growth and production of benthic macrofauna. Marine Environmental Research 9, 61-110.
- Rex M.A., Etter R.J., Morris J.S., Crouse J., McClain C.R., Johnson N.A., Stuart C.T., Deming J.W, Thies R. & Avery R. 2006. Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Marine Ecology Progress Series 317, 1-8.
- Romero-Wetzel M.B. & Gerlach S.A. 1991. Abundance, biomass, size-distribution and bioturbation potential of deep- sea macrozoobenthos on the Voring Plateau. Meeresforschung 33, 247-26.
- Rosenberg R. 1995. Benthic marine fauna structured by hydrodynamic processes and food availability. Netherlands Journal of Sea Research 34, 303-317.
- Sakshaug E. 2004. Primary and secondary production in the Arctic seas. In R. Stein & R.W. Macdonald (eds.): The Arctic organic carbon cycle. Pp. 57-82. Heidelberg: Springer.
- Shirayama Y. & Horikoshi M. 1989. Comparison of the benthic size structure between sublittoral, upper-slope and deep- sea areas of the western Pacific. International Review of Hydrobiology 74, 1-13.
- Sibuet M., Lambert C.E., Chesselet R. & Laubier L. 1989. Density of the major size groups of benthic fauna and trophic input in deep basins of the Atlantic Ocean. Journal of Marine Research 47, 851-867.
- Soltwedel T. 2013. The expedition of the research vessel “Polar- stem " to the Arctic in 2012 (ARK-XXVII/2). Berichte zur Polar- und Meeresforschung 658. Bremerhaven: Alfred Wegener Institute for Polar and Marine Research.
- Soltwedel T, Bauerfeind E., Bergmann M., Budaeva N., Hoste E., Jaeckisch N., von Juterzenka K., Matthiessen J., Mokievsky V., Nothig E., Queric N., Sablotny B., Sauter E., Schewe I., Urban-Malinga B., Wegner J., Wlodarska- Kowalczuk M. & Klages M. 2005. HAUSGARTEN: multidisciplinary investigations at a deep-sea, long-term observatory in the Arctic Ocean. Oceanography 18, 46-61.
- Soltwedel T., Mokievsky V. & Schewe I. 2000. Benthic activity and biomass on the Yermak Plateau and in adjacent deep- sea regions northwest of Svalbard. Deep-Sea Research Part I 47, 1761-1785.
- Steimle F.W 1985. Biomass and estimated productivity of the benthic macrofauna in the New York bight: a stressed coastal area. Estuarine, Coastal and Shelf Science 21, 539-554.
- Stein R. & Fahl K. 1997. Wissenschaftlicher Fahrtbericht uber die Arktis-Expedition ARK-XIII/2 von 1997 mit FS “Polarstem". (Scientific cruise report of the Arctic expedition ARK-XIII/2 of RV “Polarstem" in 1997.) Berichte zur Polarforschung 255. Bremerhaven: Alfred Wegener Institute for Polar and Marine Research.
- Thatje S. & Mutschke E. 1999. Distribution of abundance, biomass, production and productivity of macrozoobenthos in the sub-Antarctic Magellan Province (South America). Polar Biology 22, 31-37.
- Thiel H. 1975. The size structure of the deep-sea benthos. Internationale Revue der Gesamten Hydrobiologie 60, 575-606.
- Thistle D. 2003. The deep-sea floor: an overview. In P.A. Tyler (ed.): Ecosystems of the world 28. Pp. 5-37. Amsterdam: Elsevier Science.
- Wassmann P., Duarte C.M., Agusti S. & Sejr M.K. 2011. Footprints of climate change in the Arctic marine ecosystem. Global Change Biology 17, 1235-1249.
- Wassmann P., Slagstad D. & Ellingsen I. 2010. Primary production and climatic variability in the European sector of the Arctic Ocean prior to 2007: preliminary results. Polar Biology 33, 1641-1650.
- Wei C.L., Rowe G.T., Escobar-Briones E., Boetius A., Soltwedel T., Caley M.J., Soliman Y., Huettmann F., Qu F., Yu Z., Pitcher C.R., Haedrich R.L., Wicksten M.K., Rex M., Baguley J.G., Sharma J., Danovaro R., MacDonald I.R., Nunnally C.C., Deming J.W, Montagna P., Levesque M., Weslawski J.M., Wlodarska-Kowalczuk M., Ingole B.S., Bett B.J., Billett D.S.M., Yool A., Bluhm B.A., Iken K. & Narayanaswamy B.E. 2010. Global patterns and predictions of seafloor biomass using random forests. PLoS One 5(12), e!5323. doi: 10.1371/joumal.pone.0015323.
- Wildish D.J., Peer D.L. & Greenberg D.A. 1986. Benthic macrofaunal production in the Bay of Fundy and the possible effects of a tidal power barrage at Economy Point- Cape Tenny. Canadian Journal of Fisheries and Aquatic Sciences 43, 2410-2417.






