The Cingulate Cortex and Human Memory Processes


Published: Dec. 1, 2012
Latest article update: Dec. 21, 2022
Abstract
This study presents data from a magnetic-resonance morphometric (MRMM) analysis of the main regions of the cingulate cortex (in both hemispheres) and their role in memory processes in a group of healthy, females of older age. The results demonstrate a statistically reliable correlation between overall performance and the type of errors in different neuropsychological memory tests and the relative size of these regions. The discovered pattern of correlations can be explained by hypothesizing the reciprocal functional influence of the two major areas of the cingulate cortex – its anterior and posterior dorsal parts – on performance in neuropsychological memory tests.
Keywords
Cingulate cortex, cingulate gyrus, memory, human neuropsychology, magnetic-resonance morphometric analysis
The cingulate cortex (the cortex of the cingulate gyrus) is a human brain structure that has recently become the subject of many cognitive and neurocognitive studies. The cingulate cortex is situated in the medial surface of the brain between the corpus callosum and the cingulate sulcus. As a part of the limbic system, the cingulate cortex receives afferent signals fr om the anterior nuclei of the thalamus, while efferent signals fr om the cingulate cortex are transferred to the parahippocampal gyrus and then to the hippocampus. Furthermore, the cingulate cortex has many bilateral connections with the frontal, temporal, and occipital cortex of both hemispheres of the brain.
According to cerebral cytoarchitectonics, the cingulate cortex is divided into seven Brodmann areas–areas 23, 24, 26, 29, 30, 31. However, due to the difficulty of visual separation of these areas on tomographic images, the cingulate cortex is divided into three bigger areas–the anterior cingulate cortex (Brodmann areas 24 and 33), the posterior cingulate cortex (Brodmann areas 23 and 31), and the retrosplenial cingulate cortex (Brodmann areas 26, 29, and 30). In addition, sometimes the posterior cingulate cortex is divided into ventral (Brodmann area 23) and dorsal (Brodmann area 31) parts (Figure 1).
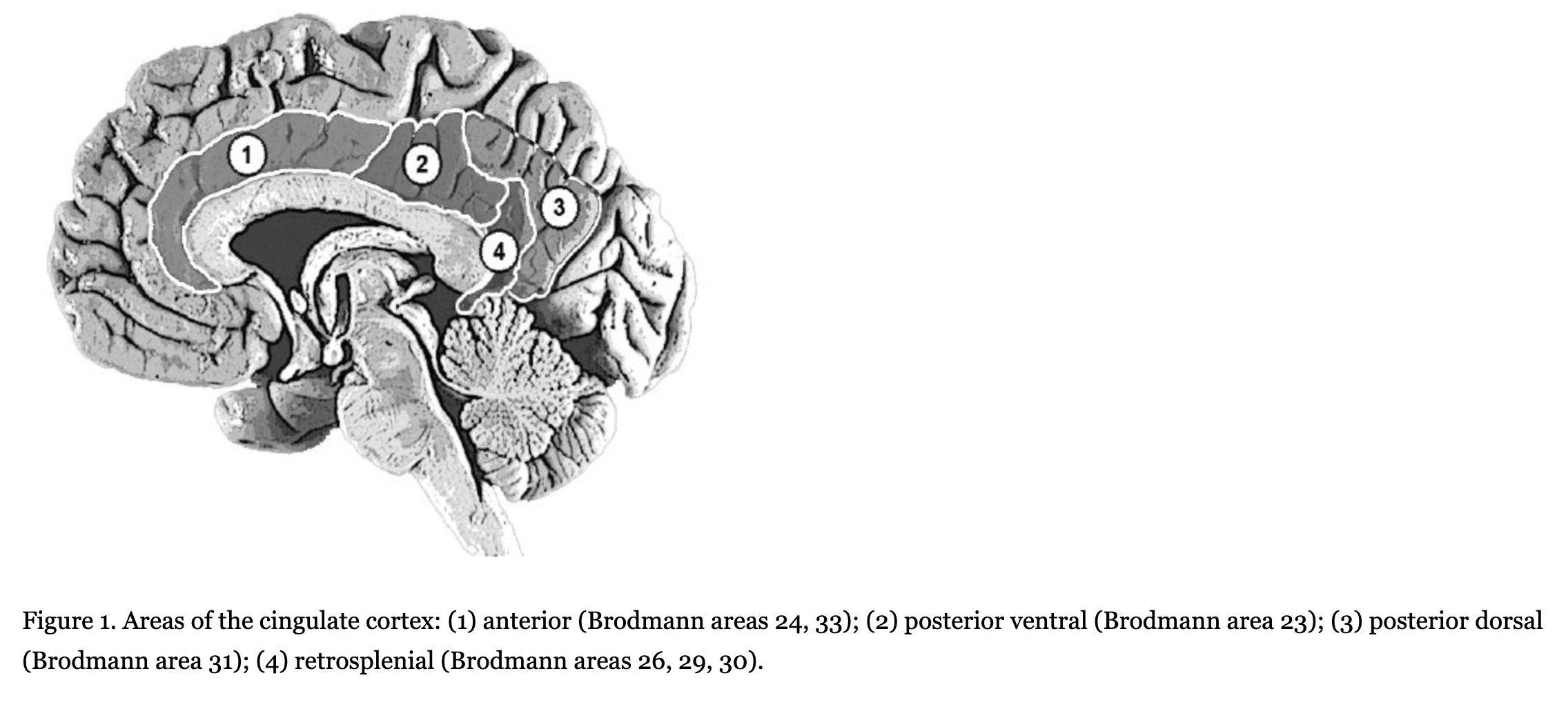
The functional role of the cingulate cortex is still being discussed, despite the large number of studies and hypotheses. Most studies concern the anterior cingulate cortex. For example, many studies show that the activity of the anterior cingulate cortex increases while subjects solve tasks involving cognitive control (such as error detection, conflict monitoring, and/or dual tasks for attention and tests for the executive component of working memory). Clinical records of lesions on this brain area in humans and experimental data in animals also show that damage to the posterior cingulate cortex results in trouble solving cognitive control tasks (Botvinick, Cohen, & Carter, 2004; Carter & van Veen, 2007; Hyafil, Summerfield, & Koechlin, 2009; Kerns et al., 2004; Lenartowicz & McIntosh, 2005; Otsuka, Osaka, Morishita, Kondo, & Osaka, 2006; Posner, Rueda, & Kanske, 2007; Rushworth, Hadland, Gaffan, & Passingham, 2003). Morphometric analysis has revealed the decreased size of the anterior cingulate cortex in subjects with schizophrenia (Choi et al., 2005; Koo et al., 2008; Mitelman, Shihabuddin, Brickman, Hazlett, & Buchsbaum, 2005).
Far less is known about the posterior cingulate cortex. According to the PubMed data base, there are almost six times fewer references to it in published works than there are to the anterior cingulate cortex. It is known that the posterior cingulate cortex activates in healthy humans when they are retrieving memories from autobiographical episodic memory (Maddock, Garrett, & Buonocore, 2001). However, if information is retrieved from episodic memory but not from autobiographical memory, this area of the brain remains inactivated (Ries et al., 2006). Also, some studies have revealed that the posterior cingulate cortex activates during recognition of familiar words, objects, and places (Heun et al., 2006; Sugiura, Shah, Zilles, & Fink, 2005). The posterior cingulate cortex in the macaque brain contains “risk neurons,” which activate if the monkey makes a risky choice; furthermore, activation increases with an increase in risk (McCoy & Platt, 2005). Electrical stimulation of these neurons resulted in decreased risky decision making in a following subtest (Hayden, Nair, McCoy, & Platt, 2008).
Published works about the functional role of the retrosplenial cingulate cortex are even less common than those of the posterior cingulate cortex. It is known that damage to this brain region causes serious anterograde amnesia (Kim et al., 2007; Oka et al., 2003). Other studies show that the retrosplenial cingulate cortex is involved in emotional processes; for example, this area activates when the subject perceives emotionally significant words (Cato et al., 2004). This area of the brain is also involved in spatial navigation; the hippocampus or the parahippocampal gyrus or both activate if one is memorizing new routes in three-dimensional space, wh ereas navigation in familiar places causes activation of the retrosplenial cingulate cortex (Epstein, 2008; Epstein, Parker, & Feiler, 2007; Iaria, Chen, Guariglia, Ptito, & Petrides, 2007). Ino and colleagues (2007) described the case of a patient with a hemorrhage in the left retrosplenial area who lost his spatial orientation but could still solve tasks for spatial thinking.
Recent studies of the involvement of different brain regions in cognitive processes use magnetic-resonance morphometric analysis. For example, some studies show that the size of the hippocampus and its subdivisions correlates with memory processes (Hackert et al., 2002; Maguire, Woollett, & Spiers, 2006; Sozinova et al., 2008; Vartanov et al., 2009). Nevertheless, morphometric analysis of the involvement of the cingulate cortex in cognitive processes has been used previously only in clinical studies. This study is the first attempt to use the method of magneticresonance morphometric analysis in order to reveal the role of the main cingulate-cortex subdivisions of both hemispheres in the memory processes of healthy subjects of older age.
Method
Participants
Participants in the study were 29 right-handed women between the ages of 60 and 76 (67.7 +/- 5.1 years old) with no previous brain trauma, strokes, psychological or neurological diseases.
Procedure
The study consisted of two parts, an MRI study and a neuropsychological assessment.
During the MRI study all participants were scanned on a 3.0 Tesla Phillips Achieva scanner; T1 three-dimensional images of the brain were received. On sagittal slices of the images (standardized in each direction) we highlighted areas that included the cingulate cortex of both hemispheres. In each hemisphere we divided the cingulate cortex into four visually separable regions–anterior (Brodmann areas 24, 33), posterior ventral (Brodmann area 23), posterior dorsal (Brodmann area 31), and retrosplenial (Brodmann areas 26, 29, 30). We calculated absolute areas of the surface of these brain regions (in mm^2) by summarizing all pixels from each cingulate cortex subdivision.
The neuropsychological part of the study included Luria’s neuropsychological assessment of cognitive processes as modified by Glozman (1999) to allow quantitative analysis of memory processes. The following tests were used: immediate and delayed recall of 10 words and of two groups of words and sentences, a semantic-coding test (memorizing 12 words by including them in random sentences and recalling them after 10 minutes of heterogenic interference), visual-memory tests (recalling simple and complex geometric objects), visuospatial-memory tests (copying geometric figures and their delayed recall), associative-memory tests (memorizing pairs of words, recall of the second word after hearing the first one). For each memory type we defined memory capacity (total of recalled elements), permanency (total of delayed recall), and memory errors. We separated several kinds of errors, such as replacement of one element with another one, confabulations (inclusion of new elements), contaminations (mixture of several elements), perseverations (recalling a single element more than once), and sequence errors.
The data were statistically analyzed by calculating nonparametric correlations (Spearman correlation coefficient) between individual behavioral and anatomical measurements. Further analysis included only statistically relevant correlations (p < 0.05).
Results
Because of the rather homogeneous sample group, no relevant correlations between subjects’ age and cingulate cortex size in both hemispheres were revealed. We also did not find any correlations between age and the sizes of the separate areas of the cingulate cortex. However, we discovered relevant correlations between cingulate cortex size and neuropsychological tests results. Moreover, these correlations varied in different cingulate cortex areas. Overall results for the anterior cingulate cortex (Brodmann area 24) of both hemispheres are summarized in Table 1.
Data in Table 1 reveal the correlation of memory-test results with the size of the anterior cingulate cortex. For example, the size of the left cingulate cortex correlates negatively with the number of confabulations in the semantic-coding and word-recall tests, so the bigger the surface of this part of the brain, the greater the recall of previous stimuli. Furthermore, the right anterior cingulate cortex correlates negatively with the total amount of verbal and, especially, nonverbal recall, so the bigger the surface of this brain area, the worse the recall of previous stimuli. Results of the visual-memory test tend to correlate with the size of the right anterior cingulate cortex, while semantic-coding and digit-recall tests correlate with the size of the left anterior cingulate cortex.
Table 1.
Overall Correlations Between Memory-Test Results and the Size of the Anterior Cingulate Cortex
| Left hemisphere | Right hemisphere | Both hemispheres |
Total of confabulations in semanticcoding test | -0.51 | - | - |
Total of confabulations in immediate recall of 10 words | - | - | -0.32 |
Total recall of 10 words | - | -0.32 | -0.33 |
Total recall in visual-memory test | - | -0.37 | - |
We also discovered relevant correlations between neuropsychological test results and the size of the ventral posterior cingulate cortex (Brodmann area 23). It is obvious from Table 2 that the size of this brain region in the right hemisphere correlates negatively with the number of errors in semantic-coding tests. However, in subjects with relatively big left ventral areas frequent sequence errors and perseverations occurred in both verbal and nonverbal tests.
Table 2.
Overall Correlations Between Memory-Test Results and the Size of the Ventral Posterior Cingulate Cortex
| Left hemisphere | Right hemisphere | Both hemispheres |
Total of confabulations in semanticcoding test | - | -0.40 | - |
Total recall in visual-memory test | -0.44 | - | - |
Sequence errors in recall of two groups of words and sentences | 0.32 | - | - |
Sequence errors in visual-memory test | 0.41 | - | - |
Sequence errors in digit recall | 0.37 | - | 0.41 |
Perseveration errors in recall of two groups of words and sentences | 0.33 | - | - |
Perseveration errors in visual-memory test | 0.40 | - | - |
Table 3 illustrates neuropsychological memory-test correlations with the size of the posterior dorsal cingulate cortex (Brodmann area 31); more correlations were revealed for this brain region than for the other ones. The size of the posterior dorsal cingulate cortex correlates negatively with verbal and nonverbal memory capacity; it also increases the influence of interfering factors and the number of confabulations in word-recall tests. Furthermore, it correlates negatively with the number of sequence errors in visual-memory tests.
Table 3
Overall Correlations Between Memory-Test Results and the Size of the Posterior Dorsal Cingulate Cortex
| Left hemisphere | Right hemisphere | Both hemispheres |
Total of confabulations in group recall test | 0.44 | - | - |
Sequence errors in visual-memory test | - | -0.46 | -0.41 |
Total recall of 10 words (before interfering stimuli) | -0.39 | - | -0.39 |
Total recall of 10 words (after interfering stimuli) | -0.49 | - | - |
Total recall of groups of words and sentences | -0.45 | - | - |
Total recall in visual-memory test | -0.45 | - | -0.41 |
Total recall in visual-memory test (after interfering stimuli) | -0.41 | - | -0.38 |
Finally, we found few relevant correlations between neuropsychological test results and the size of the retrosplenial cingulate cortex (Brodmann areas 26, 29, and 30). The results for left and right hemispheres are shown in Table 4.
Table 4
Overall Correlations Between Memory-Test Results and the Size of the Retrosplenial Cingulate Cortex
| Left hemisphere | Right hemisphere | Both hemisphere |
Total of contaminations in group recalls | - | -0.42 | -0.43 |
Total recall in visual-memory test | - | -0.39 | -0.53 |
Total recall in visual-memory test (after interfering stimuli) | -0.38 | - | -0.45 |
Table 4 reveals no relevant differences in left and right hemispheres. The increased size of this brain region correlates with a decrease in contaminations but also with a severe decrease in the number of recalled elements in visual-memory tests. This decrease remains independent of the influence of interfering stimuli.
Discussion
Results of this study show that the quantitative comparison of neuropsychological memory-test results with the anatomical structure of the cingulate cortex is meaningful. Increased size of several areas of the cingulate cortex correlates with a decrease in the number of errors in memory tests. However, a decrease in the number of errors not only does not improve overall memory performance but actually tends to deteriorate total recall.
Revealed correlations can be summarized under the concept of an anteroposterior gradient of cortical function development and interhemispheric functional differences (Krotkova & Velichkovsky, 2008; Velichkovsky, 2006). Being evolutionarily younger, anterior regions of the cortex correspond less with particular modal effects and more with semantic information processing; in this study, this correspondence results in contamination suppression. Furthermore, left-hemisphere structures reveal more sufficient correlations with verbal-test results in comparison with right-hemisphere structures. Thus, there is a negative correlation between the size of the anterior cingulate cortex and the number of confabulations in semantic-coding tests; this correlation appears to be an important characteristic of the integrity of prefrontal functions (see Turner, Cipolotti, Yousry, & Shallice, 2008). What seems to be rather counterintuitive in such correlations between test results and the size of most cingulate cortex areas can be explained by the fact that the cingulate cortex is usually referred to as the central controller of cognitive activity because it filters irrelevant information.
The dorsal cingulate cortex (Brodmann area 31), however, has controversial qualities, as its size correlates more with memory-test results than the other cingulate cortex areas and also being the only area that correlates positively with the number of confabulations.
We assume that the role of the anterior cingulate cortex in memory functioning is to filter irrelevant information in order to protect memory processes from interfering stimuli. In signal-detection theory, it can be called a filtration and noise-suppression system. This filtering explains why the overdeveloped anterior cingulate cortex correlates with a decrease in memory errors, such as confabulations, but also with a decrease in overall recall: conservative decision-making criteria filter the noise and lower the number of “false alarms” as well as lowering the number of “hits.” On the contrary, the posterior dorsal cingulate cortex detects potentially useful information, thus generating potential signals in the noise. Generally, we suggest, the cingulate cortex consists of two basic subsystems that have a reciprocal influence in neuropsychological memory- test performance and, possibly, in other cognitive processes.
This explanation corresponds with the results of other studies. If the anterior cingulate cortex is a noise-suppressing system, it becomes clear why this brain region activates during filtering tasks. It also applies to information recall because this process requires filtering and denying some false and uncertain memories, imagination, and so forth. This filtering explains the activity of the anterior cingulate cortex in information recall from spatial (Teixeira, Pomedli, Maei, Kee, & Frankland, 2006), episodic (Herrmann et al., 2001), working (Schoning et al., 2009), verbal-declarative (Bremner, Vythilingam, Vermetten, Vaccarino, & Charney, 2004), and emotional (Tang et al., 2005) memory. Based on our hypothesis of the cingulate-cortex functions, we can suggest that confabulations occur while the functioning of this brain region deteriorates. Some studies show that atrophy of the anterior cingulate cortex, such as in Alzheimer’s disease, can cause severe confabulations (Lee et al., 2009); this type of atrophy is also seen in schizophrenics, who suffer from memory errors such as confabulations (Choi et al., 2005; Koo et al., 2008; Mitelman et al., 2005). The idea that the posterior dorsal cingulate cortex (Brodmann area 31) extracts signal from noise is supported by other studies’ results as well. For example, it can explain activation of the posterior cingulate cortex in the recognition of familiar words, objects, or places (Heun et al., 2006; Sugiura et al., 2005).
The present study also revealed a functional transection of anatomically close brain regions. This finding can be again explained by the gradient principle described above. For example, the posterior ventral cingulate cortex (Brodmann area 23), being situated on the transection of two reciprocal systems, has fewer expressed properties than either of them. Overdevelopment of this brain region corresponds with the increase in the number of sequence errors and perseverations, as well as with the successful suppression of semantic errors, confabulations, and contaminations. It is also important to study the dorsoventral gradient of brain-function differences. For the anterior cingulate cortex, the specialization of different regions along the dorsoventral gradient may change from information processing under the condition of cognitive conflict to information processing under the condition of affective-emotional conflict (Posner et al., 2007; Pozner, 2008).
Acknowledgments
This study was supported by grant № 11-06-00343-а from the Russian Foundation for Basic Research (RFBR).
References
- Botvinick, M.M., Cohen, J.D., & Carter, C.S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Science, 8, 539-546.
- Bremner, J.D., Vythilingam, M., Vermetten, E., Vaccarino, V., & Charney, D.S. (2004). Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. American Journal of Psychiatry, 161(4), 637-645.
- Carter, C.S., & van Veen, V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7, 367-379.
- Cato, M.A., Crosson, B., Gokcay, D., Soltysik, D., Wierenga, C., Gopinath, K., Briggs, R.W. (2004). Processing words with emotional connotation: An fMRI study of time course and laterality in rostral frontal and retrosplenial cortices. Journal of Cognitive Neuroscience, 16(2), 167-177.
- Choi, J.S., Kang, D. H., Kim, J.J., Ha, T.H., Roh, K.S., Youn, T., & Kwon, J.S. (2005). Decreased caudal anterior cingulate gyrus volume and positive symptoms in schizophrenia. Psychiatry Research, 139(3), 239-247.
- Epstein, R.A. (2008). Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Science, 12(10), 388-396.
- Epstein, R.A., Parker, W.E., & Feiler, A.M. (2007). Wh ere am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. Journal of Neuroscience, 27(23), 6141-6149.
- Glozman, J.M. (1999). The quantitative evaluation of neuropsychological assessment data. Moscow: CLP.
- Hackert, V.H., den Heijer, T., Oudkerk, M., Koudstaal, P.J., Hofman, A., & Breteler, M.M. (2002). Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage, 17(3), 1365-1372.
- Hayden, B.Y., Nair, A.C., McCoy, A.N., & Platt, M.L. (2008). Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron, 60(1), 19-25.
- Herrmann, M., Rotte, M., Grubich, C., Ebert, A.D., Schiltz, K., Munte, T.F., & Heinze, H.J. (2001). Control of semantic interference in episodic memory retrieval is associated with an anterior cingulate-prefrontal activation pattern. Human Brain Mapping, 13(2), 94-103.
- Heun, R., Freymann, K., Erb, M., Leube, D.T., Jessen, F., Kircher, T.T., & Grodd, W. (2006). Successful verbal retrieval in elderly subjects is related to concurrent hippocampal and posterior cingulate activation. Dementia and Geriatric Cognitive Disorders, 22(2), 165-172.
- Hyafil, A., Summerfield, C., & Koechlin, E. (2009). Two mechanisms for task switching in the prefrontal cortex. Journal of Neuroscience, 29, 5135-5142.
- Iaria, G., Chen, J. K., Guariglia, C., Ptito, A., & Petrides, M. (2007). Retrosplenial and hippocampal brain regions in human navigation: Complementary functional contributions to the formation and use of cognitive maps. European Journal of Neuroscience, 25(3), 890-899.
- Ino, T., Doi, T., Hirose, S., Kimura, T., Ito, J., & Fukuyama, H. (2007). Directional disorientation following left retrosplenial hemorrhage: A case report with fMRI studies. Cortex, 43(2), 248-254.
- Kerns, J.G., Cohen, J.D., MacDonald, A.W., Cho, R.Y., Stenger, V.A., & Carter, C.S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303, 1023-1026.
- Kim, J.H., Park, K.Y., Seo, S.W., Na, D.L., Chung, C.S., Lee, K.H., & Kim, G.H. (2007). Reversible verbal and visual memory deficits after left retrosplenial infarction. Journal of Clinical Neurology, 3(1), 62-66.
- Koo, M.S., Levitt, J.J., Salisbury, D.F., Nakamura, M., Shenton, M.E., & McCarley, R.W. (2008). A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Archives of General Psychiatry, 65(7), 746-760.
- Krotkova, O.A., & Velichkovsky, B.M. (2008). Interhemispheric differences in higher gnostical regions of the brain. In Computers, brain and cognition: Advances of cognitive sciences. (pp. 107-132). Moscow: Nauka.
- Lee, E., Kinomura, S., Meguro, K., Akanuma, K., Meguro, M., & Fukuda, H. (2009). Confabulations on episodic and semantic memory questions are associated with different neurologic backgrounds in Alzheimer disease. Cognitive and Behavioral Neurology, 22(2), 81-88.
- Lenartowicz, A., & McIntosh, A.R. (2005). The role of anterior cingulate cortex in working memory is shaped by functional connectivity. Journal of Cognitive Neuroscience, 17(7), 1026-1042.
- Maddock, R.J., Garrett, A.S., & Buonocore, M.H. (2001). Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience, 104(3), 667-676.
- Maguire, E.A., Woollett, K., & Spiers, H.J. (2006). London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus, 16(12), 1091-1101.
- McCoy, A.N., & Platt, M.L. (2005). Risk-sensitive neurons in macaque posterior cingulate cortex. Nature Neuroscience, 8(9), 1220-1227.
- Mitelman, S.A., Shihabuddin, L., Brickman, A.M., Hazlett, E.A., & Buchsbaum, M.S. (2005). Volume of the cingulate and outcome in schizophrenia. Schizophrenia Research, 72(2-3), 91-108.
- Oka, Y., Maeshima, S., Morita, S., Ishida, K., Kunimoto, K., Yoshida, M., & Ueyoshi, A. (2003). [A case of amnesia caused by a subcortical hematoma in the left retrosplenial region]. No Shinkei Geka, 31(3), 289-295.
- Otsuka, Y., Osaka, N., Morishita, M., Kondo, H., & Osaka, M. (2006). Decreased activation of anterior cingulate cortex in the working memory of the elderly. Neuroreport, 17(14), 1479-1482.
- Posner, M.I., Rueda, M.R., & Kanske, P. (2007). Probing the mechanisms of attention. In J.T. Cacioppo, L. G. Tassinary, & G.G. Berntson (Eds.), Handbook of psychophysiology (pp. 410-432). New York: Cambridge University Press.
- Posner, M. (2008). Human attention systems development. In Computers, brainand cognition: Advances of cognitive sciences (pp. 93-106). Moscow: Nauka.
- Ries, M.L., Schmitz, T.W., Kawahara, T.N., Torgerson, B.M., Trivedi, M.A., & Johnson, S.C. (2006). Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage, 29(2), 485-492.
- Rushworth, M.F., Hadland, K.A., Gaffan, D., & Passingham, R.E. (2003). The effect of cingulate cortex lesions on task switching and working memory. Journal of Cognitive Neuroscience, 15(3), 338-353.
- Schoning, S., Zwitserlood, P., Engelien, A., Behnken, A., Kugel, H., Schiffbauer, H., & Konrad, C. (2009). Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Human Brain Mapping, 30(9), 2746-2756.
- Sozinova, E.V., Kozlovskiy, S.A., Vartanov, A.V., Skvortsova, V.B., Pirogov, Y.A., Anisimov,
- N.V., & Kupriyanov, D.A. (2008). The role of hippocampus parts in verbal memory and activation processes. International Journal of Psychophysiology, 69(3), 312.
- Sugiura, M., Shah, N.J., Zilles, K., & Fink, G.R. (2005). Cortical representations of personally familiar objects and places: Functional organization of the human posterior cingulate cortex. Journal of Cognitive Neuroscience, 17(2), 183-198.
- Tang, J., Ko, S., Ding, H.K., Qiu, C.S., Calejesan, A.A., & Zhuo, M. (2005). Pavlovian fear memory induced by activation in the anterior cingulate cortex. Molecular Pain, 1, 6.
- Teixeira, C.M., Pomedli, S.R., Maei, H.R., Kee, N., & Frankland, P.W. (2006). Involvement of the anterior cingulate cortex in the expression of remote spatial memory. Journal of Neuroscience, 26(29), 7555-7564.
- Turner, M. S., Cipolotti, L., Yousry, T. A., & Shallice, T. (2008). Confabulation: Damage to a specific inferior medial prefrontal system. Cortex, 44(6), 637-648.
- Vartanov, A.V., Kozlovskiy, S.A., Skvortsova, V.B., Sozinova, E.V., Pirogov, Y.A., Anisimov, N.V., & Kupriyanov, D.A. (2009). Pamjat’ cheloveka i anatomicheskie osobennosti gippokampa [Human memory and anatomic features of the hippocampus]. Vestnik Moskovskogo universiteta [Bulletin of Moscow University], Series 14 – Psychology, 4, 3-16.
- Velichkovsky, B.M. (2006). Kognitivnaja nauka: Osnovy psikhologii poznanija [Cognitive science: Foundations of epistemic psychology], vol. 2. Moscow: Smysl; Akademija.





