Use of multiple representations in developing preservice chemistry teachers’ understanding of the structure of matter


Published: Jan. 10, 2013
Latest article update: Jan. 10, 2023
Abstract
The purpose of this study was to examine the changes in 19 preservice chemistry teachers’ understandings of the structure of matter, including the aspects of the physical states of matter, the physical composition of matter, and the chemical composition of matter, before, immediately after, and months after they received a specific instruction. The one-group pre, post, and delayed posttest design was used, and participants’ understandings before, immediately after, and months after the instruction were assessed using the same “three part particulate drawing” classification question constructed by Sanger (2000). Collected data were analyzed according to both the number of scientifically appropriate classifications, and the types and nature of scientifically inappropriate classifications made by preservice teachers. The results of these two analyses were quite parallel to each other and showed that this specific instruction promoted the development of participants’ scientific understandings of the structure of matter. It should be noticed that while the effect of the instruction appeared extremely positive based on the results of the statistical analyses which solely compared the number of scientifically appropriate classifications, it was reflected more accurately after the participants’ scientifically inappropriate classifications of the structure of matter were analyzed more thoroughly. It was also found that although some scientifically inappropriate classifications were changed to scientifically appropriate ones following the instruction, some of them reverted back to their initial status months after the instruction.
Keywords
Teacher education, multiple representations in chemistry, preservice chemistry teachers, conceptual understanding
Introduction
Conceptual understandings and the associated alternative conceptions in chemistry have occupied researchers' attention for more than 30 years (Duit, 2009; Garnett, Garnett, & Hackling, 1995). Previous research has shown that students at all grade levels encounter conceptual difficulties even with basic chemistry concepts, and they often develop conceptions which differ from those accepted by the scientific community (Kind, 2004; Taber, 2002). Some of these conceptions derive from individuals' direct or indirect observation of, and spontaneous everyday interaction with, the natural world around them (Driver, Squires, Rushworth, & Wood-Robinson, 1994). Other sources of these conceptions might be textbook misrepresentations, misleading everyday language, and even the act of teaching itself due to inappropriate instructional materials or teachers' own alternative conceptions (Adbo & Taber, 2009; Duit & Treagust, 1995; Lin, Cheng, & Lawrenz, 2000).
The extent of teachers' subject matter knowledge and the nature of their alternative conceptions may affect how their students understand the concepts. According to Shulman (1987), in order to be able to teach all students, teachers need to hold deep conceptual understandings of the subject matter, but several research studies have reported that preservice and inservice teachers have diverse nonscientific conceptions about fundamental concepts of chemistry (Azizoglu, Alkan, & Geban, 2006; Baneijee, 2001; Calik & Ayas, 2005; Canpolat, 2006; Canpolat, Pinarbasi, & Sozbilir, 2006; Gabel, Samuel, & Hunn, 1987; Ginns & Watters, 1995; Haidar, 1997; Kokkotas, Vlachos, & Koulaidis, 1998; Kruse & Roehrig, 2005; Lin et al., 2000; Ozden, 2009; Taber & Tan, 2011; Tan & Taber, 2009; Valanides, 2000). Moreover, some of these studies have identified similarities between students' and teachers' alternative conceptions (Calik & Ayas, 2005; Kokkotas et al., 1998; Lin et al., 2000; Valanides, 2000). This indicates a potentially crucial problem, in that if teachers do not hold scientific understandings, they may pass these alternative conceptions on to their students (Kruse & Roehrig, 2005; Taber & Tan, 2011). Teachers with limited subject matter knowledge may not be aware of students' alternative conceptions and may not be able to offer viable explanations to their students (Jarvis, McKeon, & Taylor, 2005).
The existing literature (Calik & Ayas, 2005; Haidar, 1997; Kokkotas et al., 1998; Kruse & Roehrig, 2005; Lin et al., 2000; Taber & Tan, 2011; Valanides, 2000) consistently recommended addressing students' alternative conceptions with proper instructional activities and making teachers, particularly preservice teachers, aware of their own alternative conceptions through specific instructions before they go into teaching practice. Since teachers are charged with developing scientific understandings, the instruction offered for preservice science teachers in teacher education programs and for inservice teachers in workshops needs to be arranged around the idea of eliciting and building teachers' own conceptions and encouraging teachers to develop the teaching skills to continuously promote scientific conceptual understandings among students (Kruse & Roehrig, 2005; Valanides, 2000).
As a response to this call, in the present study, a specific instruction was designed to improve pre service chemistry teachers' understandings of the structure of matter. This instruction provided participants with activities to uncover and restructure their conceptions about the structure of matter. These activities involved examining and building multiple molecular representations of matter with their peers in groups, as well as discussing and reflecting on their understandings of matter.
Theoretical Background
Understanding the structure of matter is part of a targeted concept in school science curricula worldwide, for Grades 6 through 12. Students are expected to understand the structure of matter at three physical states, and they should be able to recognize and classify the elements, compounds, pure substances, and mixtures at different representational levels. Developing a scientific understanding about the structure of matter is essential for learning advanced chemistry topics (Gabel, 1993; Haidar & Abraham, 1991; Harrison & Treagust, 2002). Previous research mostly focused on identifying and describing elementary through university students' alternative conceptions about the particulate nature of matter [PNM] (e.g., Griffiths & Preston, 1992; Liu & Lesniak, 2005; Margel, Eylon, & Scherz, 2008; Novick & Nussbaum, 1978; Pozo & Gomez-
Crespo, 2005; Sanger, 2000; Stains & Talanquer, 2007a, 2007b; Williamson, Huffman, & Peck, 2004; among others). However, research into preservice teachers' conceptual understandings of the nature and structure of matter (Gabel et al., 1987; Ginns & Watters, 1995; Kokkotas et al., 1998; Valanides, 2000) is limited in number and considered only preservice elementary science teachers. There are some studies examining high school preservice chemistry teachers' conceptual understandings, but none of these were related to the nature and structure of matter, instead related to concepts of phase equilibrium (Azizoglu et al., 2006), chemical equilibrium (Baneijee, 1991), gas laws (Lin et al., 2000), ionization energy (Tan & Taber, 2009), and conservation of mass (Haidar, 1997).
Research into Preservice Teachers’ Understanding of the Nature and Structure of Matter
In order to identify preservice elementary teachers' views of the PNM, Gabel and her colleagues (1987) expected the participants to distinguish elements, compounds, mixtures, substances, solutions, solids, liquids, gases, and chemical and physical changes at the submicroscopic level. The findings from this study indicated a lack of understanding of the PNM and the existence of various alternative conceptions, such as enlargement of particles as the substance change from liquid to gas.
Ginns and Watters (1995) also conducted a study with preservice elementary teachers, and the results revealed that some participants were "unable to conceptualize the behaviour of matter at the particulate level in order to explain phenomena of the kind embodied in the question, and, therefore unwilling to use the terms atom, molecules, and even particle'' (p. 215). In addition, Valanides (2000) found that the majority of preservice elementary teachers in the sample exhibited perceptual rather than conceptual understanding of the PNM, and they had difficulties in relating the macroscopic changes to the submicroscopic occurrences (e.g., arrangement and movement of particles). These participants stated that individual particles share the observable properties of matter and combine together to produce new molecules, but they could not realize the changes in the structure and the properties of matter.
In this respect, research in science education has strongly suggested utilizing more effective teaching methods in teacher education courses in order to develop a sound understanding of fundamental chemistry concepts among preservice teachers (Calik & Ayas, 2005; Calik, Ayas & Coll, 2007; Ginns & Watters, 1995; Haidar, 1997; Kruse & Roehrig, 2005; Valanides, 2000; among others). The following section summarizes a few attempts in this direction to respond these suggestions.
Instructional Attempts to Develop Preservice Teachers’ Understandings of Chemistry Concepts
Kokkotas et al. (1998) attempted to improve preservice elementary teachers' knowledge about the PNM. In doing so, preservice teachers were confronted with actual students' responses about matter and its transformations. Preservice teachers first evaluated the students' responses, then discussed, in small groups, their opinions about these responses and possible obstacles to learning these topics, recommended appropriate teaching activities for eliminating these obstacles, and lastly re-evaluated students' responses. The results from that study showed that many participants initially tended to lack scientific understanding about the PNM, but showed substantial improvement after the instruction.
Jarvis and her colleagues (2005) specifically designed an instruction to support the preservice elementary teachers' science subject matter knowledge. In this instruction, the participants were initially encouraged to become aware of the limitations of their own knowledge. Then, experiences were provided to challenge their existing ideas and to help them reach the scientific view through discourse and practical, active, and collaborative learning. The results from the study indicated a substantial conceptual improvement among the participants following the instruction. Taylor and Coll (1997) tested the use of an analogy for addressing a common alternative conception of preservice elementary teachers regarding the dissolving process. A bridging analogy was utilized to act as a bridge between the anchoring situation (dissolving of potassium permanganate) and the target situation (dissolving of sugar). In another study, Calik et al. (2007) used conceptual change texts to overcome preservice elementary teachers' alternative conceptions about solution chemistry. Their findings showed that the use of conceptual change texts was a cost-effective and resource-effective way to improve the participants' conceptual understanding of the dissolving of solids in water.
Although all these instructions adapted by aforementioned studies targeted pre service teachers' understandings of particular concepts, none of them addressed the concepts similar to the ones dealt with in the present study. Moreover, Sanger (2000), and Stains and Talanquer (2007a) in their studies addressed the similar concepts as in this study, but they worked with university chemistry students rather than pre service chemistry teachers.
For example, Sanger (2000) developed and used the particulate drawings (which were also used in the current study) in interviews to identify the ways students classify these particulate drawings as pure substances, heterogeneous or homogeneous mixtures. Then, Sanger designed an instruction based on the results of the interviews such that he provided some macroscopic samples in test tubes and showed their submicroscopic computer-generated visuals, and students classified the given particulate representations. This was an attempt to explicitly link different representations of the given substances.
In addition, Stains and Talanquer (2007a, 2007b), without any instruction, asked the participants to classify chemical substances as elements, compounds or mixtures based on their particulate representations similar to the ones used in this study. They identified the patterns of reasoning used by undergraduate students in classifying chemical substances.
Multiple Representations in the Teaching and Learning of Chemistry
Many chemical phenomena happen at the submicroscopic level and are not accessible to direct observation (Gabel, 1998). Thus, a full understanding of chemistry requires students to "make sense of the invisible and untouchable" (Kozma & Russell, 1997, p. 949). In other words, for developing a scientific understanding of a phenomenon, students need to properly relate the three levels of representation to one another (Gilbert & Treagust, 2009; Kozma, 2003). The three levels of representation include: (a) macroscopic refers to tangible, visible, and edible aspects of matter (b) submicroscopic provides information about atomic, molecular, and kinetic aspects of matter, and (c) symbolic involves the use of symbols, formulas, and diagrams (Gabel, 1998; Johnstone, 1993). For example, the symbol of H2(g) submicroscopically refers to the diatomic molecules existing at gas state; whereas macroscopically it is a colourless and odourless gas, weighing 2 grams per mole.
Cheng and Gilbert (2009) claimed that to conceptually learn science, students need to understand the various representations of science concepts, be able to translate between different representations, as well as demonstrate a capacity to construct a representation in any form for a given purpose. Even if the simultaneous use of the three levels of representation holds the promise of promoting student learning (Ardac & Akaygun, 2005; Tasker & Dalton, 2006), learning from/with multiple level of representations can be a difficult task for students. For example, Hinton and Nakhleh (1999) found out that although all of the participants were able to identify
macroscopic changes as evidence for chemical reactions and perform algorithmic calculations involving chemical reactions, none of the students demonstrated a clear understanding of the submicroscopic nature of chemical reactions.
The cognitive theory of multirepresentational learning (Mayer, 2009) also supports the use of multiple representations for instructional purposes. Mayer (2003) claimed that "students learn more deeply from a multimedia explanation presented in words and pictures than in words alone’’ (p. 131). This is because the extent of knowledge processed on two channels is greater than that processed on one channel. In addition, using multiple representations in instruction alleviate possible cognitive load in learning complex concepts (Sweller & Chandler, 1991).
According to Mayer (2009), multirepresentational learning is based on three assumptions: dual-channel, limited-capacity, and active processing (Mayer, 2003, 2009). The pictures (e.g., static particulate drawings or dynamic particulate animations) and words (e.g., oral or textual narrations of relevant pictures) from external sources are detected through either eyes and ears, and students selectively register incoming pictures and words and transmit them to their working memory for further processing by the visual and/or the verbal channel. Students actively process the selected pictures and words by either directly organising them as verbal and visual representations, or turning either one into the other form of representation to be further processed in a different channel. After a set of selecting and organising processes, students construct a verbal and/or visual mental representation of the phenomena. Then, they build referential coherent links between the verbal and the corresponding visual mental representation as well as integrating them with the relevant aspects of existing prior knowledge from long-term memory.
To address the challenges students encounter while moving between three levels of representation, researchers suggested engaging students with dynamic, multirepresentational visualizations of invisible phenomena, and among these are technologies that include animations and simulations of phenomena with multiple levels of representation (e.g., Ardac & Akaygun, 2005; Kozma & Russell, 1997; Sanger, 2000; Tasker & Dalton, 2006; Williamson & Abraham, 1995; Yezierski & Birk, 2006). In fact, during the instruction, teachers need to help students become explicitly aware of the relationship between the expressed and represented world and connect various representations together (Hinton & Nakhleh, 1999; Tasker & Dalton, 2006). Many studies conducted with students (not preservice teachers) have suggested that the careful use of multiple representations not only offer students opportunities to improve their conceptual understanding of chemistry but also expand our understanding of how students interpret and utilize such representations for making sense of the given phenomena (Ardac & Akaygun, 2004, 2005; Chandrasegaran, Treagust, & Mocerino, 2007, 2008; Ebenezer, 2001; Sanger, 2000; Stieff, Hegarty, & Deslongchamps, 2011; Tasker & Dalton, 2006).
Purpose and Research Questions of the Study
Based on the reviewed literature, it seems that there is a lack of research which concerns about developing preservice chemistry teachers' conceptual understanding in fundamental chemistry concepts by using multiple representations during the teacher training. So different than the other studies mentioned in the previous section, this study integrated the use of multiple representations (via paper clips, play-dough, dynamic computer animations, verbal explanations, and pictorial drawings) into an instruction intended to enhance preservice chemistry teachers' understandings of the structure of matter.
The purpose of the study was to examine the changes in preservice chemistry teachers' understandings of the structure of matter, including (a) the physical states of matter, (b) the
physical composition of matter (pure substance, mixture), and (c) the chemical composition of matter (only elements, only compounds, and both) before, immediately after, and months after they received a specific instruction. The following research questions guided the study:
- How does the number of scientifically appropriate classifications of preservice chemistry teachers for each aspect of the structure of matter change from pre to post and then to delayed postinstruction?
- How do pre service chemistry teachers' scientifically inappropriate classifications for each aspect of the structure of matter change from pre to post and then to delayed postinstruction?
While the first question focused on the number of scientifically appropriate classifications before and after the instruction by using a quantitative perspective in data analysis, the second one aimed at understanding the changes in the type of scientifically inappropriate classifications after the instruction from a qualitative perspective.
Methods
Design
This is a longitudinal study of one-group quasi-experimental design with a pre, post, and delayed posttest (Campbell & Stanley, 1966; White & Arzi, 2005). This study qualifies to be a longitudinal study, as it meets the criteria of multiple measures and a long duration of time (17 months) between the first and last measure (White & Arzi, 2005).
Participants and Setting
The participants of the study consisted of 19 preservice chemistry teachers, including 10 female and 9 male. These participants were third-year students in a five-year teacher education program. All participants completed not only introductory level chemistry courses in which the aspects of the structure of matter were addressed, but also advanced chemistry courses such as inorganic chemistry, analytical chemistry, organic chemistry, etc. The study was conducted in a secondary school laboratory applications course offered by the second author of the study. In this course, the course instructor created a learning environment in which preservice teachers could gain first-hand experience with contemporary instructional approaches, as well as develop their scientific understandings of relevant topics such as the structure of matter.
Framework of the Instruction
Drawing upon the information about student learning provided by Mayer (2003, 2009), the instructional implications of three aspects of chemistry (macroscopic, submicroscopic, and symbolic) (Gabel, 1998; Johnstone, 1993), and the recommendations of several studies (Ardac & Akaygun, 2004, 2005; Chandrasegaran et al., 2007, 2008; Hinton & Nakhleh, 1999; Sanger, 2000; Stieff et al., 2011; Tasker & Dalton, 2006; Tsai, 1999) about the use of multiple representations in teaching practice, the following instruction was designed. In this instruction, multiple representational tasks, combined with collaborative group work, discussion, and self-reflection, provided pre service teachers an opportunity for generating more scientific representations of matter. Summary of the instruction can be seen in Table 1.
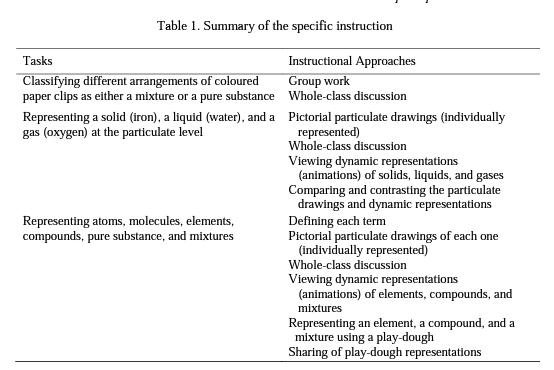
The instruction was completed in two phases and lasted 3 class periods. In the first phase, the participants engaged in a task adapted from Blake, Hogue and Sarquis (2006). They worked in groups of three or four, and each group was provided with seven zip-lock bags containing different arrangements of coloured paper clips, each of which represented either a mixture or a pure substance (element or compound). The participants identified the contents of each bag, classifying them either as a mixture or a pure substance, and then described the composition of each bag in detail. Once the participants finished this task, the instructor initiated a whole class discussion by soliciting each group's ideas about the composition of each bag (e.g., "Bag 1 represents a mixture, and it is a mixture of compounds"). Following the discussions among groups, the participants reached a consensus view about the contents of each bag.
In the second phase, the participants first represented a solid, a liquid, and a gas at the particulate level by using just closed circles Q ] without concerning about the representation of atoms, molecules, or ions; however, they considered the arrangement and spacing between the particles of a solid, a liquid, and a gas. Subsequently, the instructor initiated a discussion on the behaviour of solids, liquids, and gases at the particulate level; thus, the participants shared their representations along with their explanations regarding the behaviour of the particles of solids, liquids, and gases. Then, the participants viewed the dynamic animations of solids, liquids, and gases for about a minute (see http://www.chem.purdue.edu/gchelp/atoms/states.html) and compared with their own representations in terms of the arrangement and spacing of particles. Afterwards, the participants worked in groups, and responded the questions in activity sheets that asked them to verbally define the terms of an atom, a molecule, an element, a compound, a mixture, and a pure substance, and pictorially represented each one at the submicroscopic level by selecting a specific element, compound, mixture, and a pure substance. Then, a whole-class discussion was held concerning such terms so that each group was shared their verbal expressions of each term with the class, and students developed a consensus view about each term. Then, the participants viewed the dynamic representations of elements (including both atomic and molecular element), a compound, and a mixture for about two or three minutes (see http://www.chem.purdue.edu/gchelp/atoms/elements.html). After that, students individually represented their selected examples of each category [the ones that they represented on the paper] at the submicroscopic level using play-dough. While representing their choice for each category, students were asked to pay particular attention to the physical state of the selected element, compound, and mixture at room temperature. Once the participants completed the play-dough task, they shared their representations with their classmates and received feedback from their peers and instructor.
Data Collection
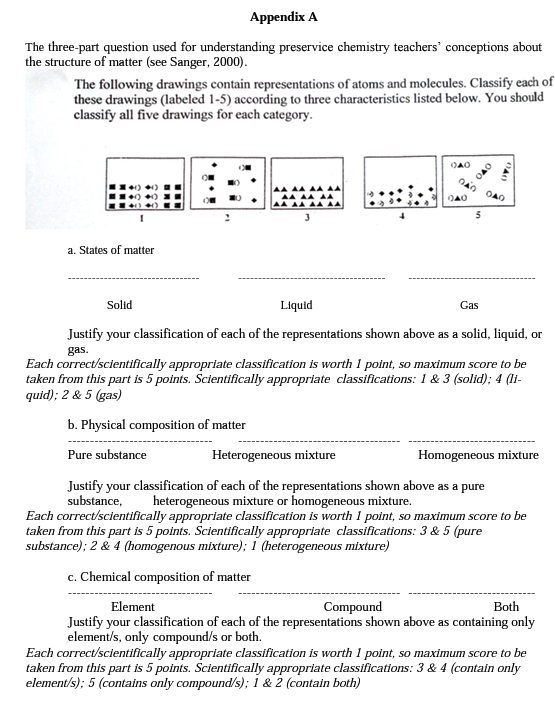
Several researchers have recently used questions that included particulate drawings instead of numerical problems because research indicated that students who are successful at solving numerical problems may not understand the concepts underlying these problems (Nurrenbem & Pickering, 1987; Sanger, 2000; Stains & Talanquer, 2007a, 2007b). Drawing upon this finding, this study assessed preservice chemistry teachers' understandings of the structure of matter before, immediately after, and 17 months after the instruction using the same "three-part particulate drawing question'’ constructed by Sanger (2000) (see Appendix A). This task presented five pictures representing five different matters at the submicroscopic (molecular) level. In the pre, post, and delayed postinstruction measurements, the participants were asked to classify each of the five given pictures according to: (a) its physical state (solid, liquid, gas), (b) its physical composition (pure substance, homogeneous mixture, heterogeneous mixture), and (c) its chemical composition (element/s only, compound/s only, and both), and to write down justifications for their classifications. The participants took the pretest at the beginning of the Autumn Semester (last week of September), and the instruction was implemented in the middle of November. The participants took the posttest after the instruction, and the delayed posttest was administered 17 months after the instruction. Each time, the participants answered "the three part particulate drawing” question within 20 minutes.
Data Analysis
For quantitative analysis of the data, one point was assigned for each scientifically appropriate classification about the given particulate drawings. The maximum score for each part of the question was five, and the possible maximum total score was 15 (see Appendix A for scoring). The total numbers of scientifically appropriate classifications for each part were added up for each participant and their total scores were calculated at three data collection instances (i.e., pre, post, and delayed posttest). Numerical data was analyzed using statistical analysis in order to detect changes due to instruction, if any. The Wilcoxon-signed ranks test was utilized to compare the participants' scores for each aspect of the structure of matter and their total scores from pre to postinstruction, from pre to delayed postinstruction, and from post to delayed postinstruction. The Wilcoxon signed-ranks test is a non-parametric statistical test for comparing two related samples or repeated measurements on a single sample to assess whether their population mean ranks differ (Gibbons, 1993). Therefore, it can be considered the non-parametric counterpart of the paired- samples t-test.
Apart from the aforementioned analysis, which took into account the scientifically appropriate classifications, the data were also analyzed in order to identify the types of
participants' scientifically inappropriate classifications at three data collection instances. In doing so, a frequency count was performed for each kind of scientifically inappropriate classifications on the pre, post, and delayed posttest. Researchers also read through the participants' written justifications for their classifications to understand their way of thinking. These written justifications provided evidence for the participants' conceptions about the nature and structure of matter. Some representative examples among these written justifications were given in the "results and discussion" section to offer the reader an idea about the participants' reasoning.
Results and Discussion
Research Question 1: How does the number of scientifically appropriate classifications of preservice chemistry teachers for each aspect of the structure of matter change from pre to post and then to delayed postinstruction?
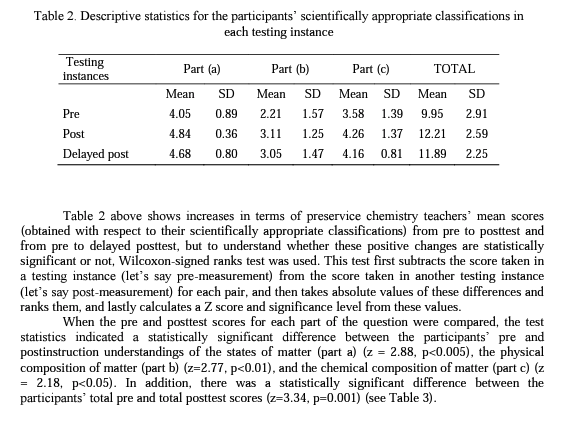
Table 2 shows the pre, post, and delayed posttest mean scores and standard deviations for each aspect of the structure of matter: (a) the physical state of matter, (b) the physical composition of matter, (c) the chemical composition of matter.
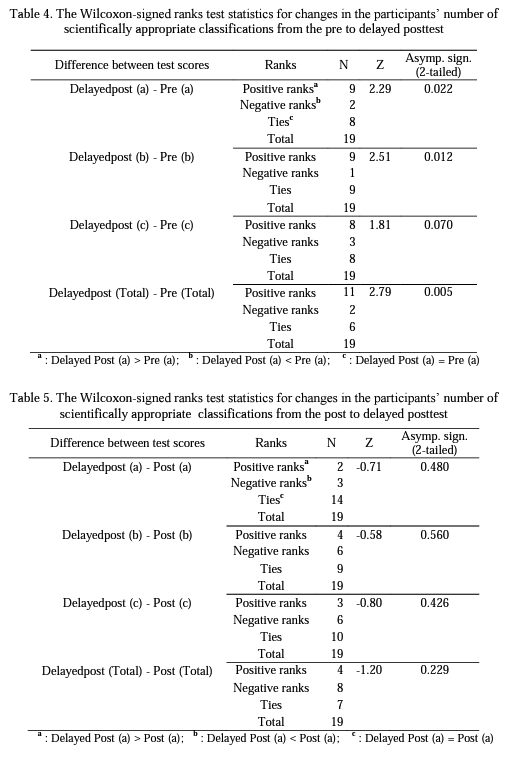
Research Question 2: How do preservice chemistry> teachers' scientifically inappropriate classifications of each aspect of the structure of matter change from pre to post and then to delayed postinstruction?
In addition to the analysis reported in the previous section which solely focuses on the number of scientifically appropriate classifications, to answer the second research question, the participants' scientifically inappropriate classifications were categorized and the frequencies for each type of scientifically inappropriate classification were determined. These inappropriate classifications concerning each aspect of the structure of matter were explained under the separate headings below.
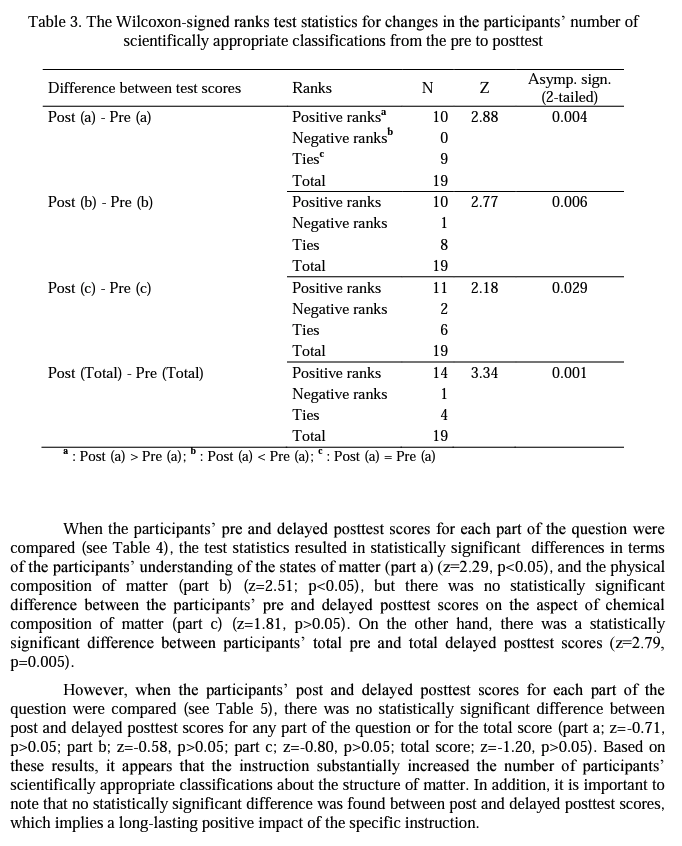
Scientifically inappropriate classifications related to “the physical states of matter”. The number of scientifically inappropriate classifications for the given particulate representations with respect to their physical states was not high (n=18) before the instruction, and the frequency of scientifically inappropriate classification cases even decreased (n=3) following the instruction (see Table 6). In the classification task, it appears that Picture 3 is the most challenging one for the participants when compared to the other pictures (see Appendix A for the pictures). Some participants failed to classify it as solid (misclassifying it as liquid) on the pretest (n=5), posttest (n=3), and delayed posttest (n=l). Moreover, the representations of gases (Picture 2 and 5) were misclassified as liquids by seven preservice teachers on the pretest and two pre service teachers on the delayed posttest. All of the preservice teachers scientifically classified these two pictures on the posttest.
The participants provided the different types of explanations to justify their classification related to the physical states of matter. While the participants were using macroscopic level evidence (e.g., “taking the shape of the container’ or “occupying the container”) in their written justifications on the pretest, many preservice teachers included submicroscopic level evidence in their explanations by taking into account “the distance between particles” and/or “the arrangement of individual particles” in the given representation on the post and delayed posttest. A participant's written justifications across three testing instances showed this change: “Pictures 2 and 5 are gases, because gases don't cover a certain area, they occupy the container” (pretest), “Pictures 2 and 5 are gases, because the molecules spread all over the container. There are much more space between the molecules, and they are randomly distributed” (posttest), “Pictures 2 and 5 are in gas form, because the particles are free from each other, they are distributed randomly, and they are everywhere in the box (delayed posttest)”.
Table 6. Frequencies of types of scientifically inappropriate classifications about the physical states of matter on the pre, post, and delayed posttest
Types of nonscientific classifications | Pre | Post | Delayed Post |
Solid (P 1) as liquid | 1 | 0 | 1 |
Gas (P 2) as liquid | 3 | 0 | 0 |
Solid (P 3) as liquid | 5 | 3 | 1 |
Liquid (P 4) as solid | 4 | 0 | 0 |
Liquid (P 4) as gas | 1 | 0 | 2 |
Gas (P 5) as liquid | 4 | 0 | 2 |
Total | 18 | 3 | 6 |
The Picture 3 was the most inappropriately classified one by the participants. This particulate drawing was misclassified as liquid, although it represented a solid. One of the participants, on the pretest, justified his classification such that "molecules are close, but there is still a distance that makes it liquid/’ It seems that this preservice teacher viewed liquids in-between the solids and gases in terms of the spacing between their atoms/molecules, and disregarded the ordered structure of solid particles. He then appropriately classified Picture 3 as being a solid following the instruction. He explained his classification, stating that "Picture 3 is solid, because the molecules are arranged regularly”.
Scientifically inappropriate classifications related to “the physical composition of matter”. Classifying the given particulate representations with respect to their physical composition — in other words, whether they were pure substances, homogeneous mixtures, or heterogeneous mixtures — was the most difficult task for the participants in all three data collection instances. Compared to the other aspects of the structure of matter, there were numerous scientifically inappropriate classifications of the physical composition of matter on the pretest (n=45) (see Table 7). Following the instruction, the number of inappropriate classification cases considerably decreased (n=34), but on the delayed posttest, the participants' scientifically inappropriate classification of the particulate representations increased again (n=40).
Many participants appropriately classified the particulate drawing (Picture 3) representing an element as pure substances, but misclassified the drawing (Picture 5) representing a compound as homogeneous mixture (n=9) and the drawings (Picture 2 and 4) representing homogeneous mixtures as heterogeneous mixtures (n=27) before the instruction (see Table 7). This finding is very much parallel with the findings of Sanger's (2000) study whose sample was university level chemistry students. Sanger indicated that "some students classified pure compounds (Picture 5) as mixtures because they contain two or more atom types, but as homogeneous because they look the same throughout the picture, and classified all mixtures (Picture 1, 2, and 4) as heterogeneous because they can see two different kinds of things in the mixture” (p.763).
In the present study, many participants were confused about homogeneous and heterogeneous mixtures at three data collection instances. For example, on the pretest, 15 (of the 19 participants) misclassified Picture 4, and 12 (of the 19 participants) misclassified Picture 2, as heterogeneous mixture, when in fact, both of these pictures represented homogeneous mixtures. The participants who classified homogenous mixtures as heterogeneous mixtures (Picture 2 and 4) generally indicated in their written justifications that these pictures had "more than one type of elements or compounds” and also they had an "irregular (or unequal) distribution of particles.”
Table 7. Frequencies of types of scientifically inappropriate classifications about the physical composition of matter on the pre, post, and delayed posttest
Types of nonscientific classifications | Pre | Post | Delayed Post |
Heterogeneous mixture (P 1) as homogeneous mixture | 6 | 6 | 4 |
Homogeneous mixture (P 2) as heterogeneous mixture | 12 | 8 | 9 |
Pure substance (P 3) as homogeneous mixture | 1 | 3 | 4 |
Homogeneous mixture (P 4) as heterogeneous mixture | 15 | 13 | 13 |
Pure substance (P 5) as homogeneous mixture | 9 | 3 | 7 |
Heterogeneous mixture (P 1) as pure substance | 2 | 1 | 3 |
Total | 45 | 34 | 40 |
A participant who inappropriately classified a pure substance (a compound) as a homogenous mixture (Picture 5) stated in his written justifications that Picture 5 "included a single type of molecule in the gas phase, so it is a homogeneous mixture'’. Thus, it might be speculated that some participants exhibited rather naive reasoning, assuming that "all mixtures are heterogeneous, and compounds are homogeneous mixtures”, previously claimed by Sanger (2000, p.766). In addition, it seems that the homogeneity property of compounds directed some participants to classify compounds as homogeneous mixtures as it was evidenced from a participants' written justification, stating that "Picture 5 is homogeneous mixture because the molecules of this substance are distributed properly in its volume”. Similarly, more than half of the university students interviewed in Stains and Talanquer's studies (2007a, 2007b) made comments that revealed their inability to differentiate between the concepts of compound and mixture.
Moreover, in the scientifically inappropriate classification of Picture 1, which is a representation of a heterogeneous mixture in the solid phase, some participants paid attention to "the ordered distribution of particles” and "the tight structure”, and this led them to categorize this heterogeneous mixture as a homogeneous mixture. A participant's written justification, "Picture 1 is a homogeneous mixture because you have a chance to take the same thing from every part of the container, they're equally arranged”, might be the result of such a reasoning.
Scientifically inappropriate classifications related to “the chemical composition of matter”. The number of scientifically inappropriate classifications associated with the chemical composition of the given particulate representations was not so high when compared to the physical composition aspect of the structure of matter [n=15 (pre), n=2 (post), n=13 (delayed post)] (see Table 8).
In this particular classification of matter task, pictures 1 and 4 were frequently misclassified. Picture 1 was misclassified by some participants as being composed of compounds only, although it was in fact composed of both an element and a compound. Some participants who responded in this way perceived each line as a big molecule of a compound, even though the squares and the other compounds in the representation were not so close and bonded to each other. A justification offered by a participant reflected such an idea, which stated that "In Picture 1, there is only one compound present, there is no element, and all atoms are connected to the other atoms, so it's a complicated compound”. A similar misperception was reported in Sanger's study (2000), and this might be considered to be a limitation of this drawing; thus, that representation (Picture 1) should be used with caution.
Table 8. Frequencies of types of scientifically inappropriate classifications about the chemical composition of matter on the pre, post, and delayed posttest
Types of nonscientific classifications | Pre | Post | Delayed Post |
Composing of both (P 1) as only compound | 5 | 1 | 6 |
Composing of both (P 2) as only compound | 2 | 0 | 0 |
Composing of only element (P 4) as both element and compound | 5 | 0 | 5 |
Composing of only element (P 4) as only compound | 3 | 1 | 2 |
Total | 15 | 2 | 13 |
Some participants found Picture 4 hard to classify. Although this drawing represented the matter containing two different elements, some participants inappropriately classified it as composing of a compound only or composing of both an element and a compound. One of the participants, on the pretest, justified his inappropriate classification such that "In Picture 4, two different types of atoms is connected, so it's a compound". This same participant appropriately classified this picture on the post and delayed posttest. His written justification on the posttest was that "Picture 4 is composed of elements, it's actually a mixture of elements" and his delayed posttest justification was very similar to this. This improvement provided an evidence for the contribution of the instruction on the participants' conceptual understandings.
Conclusions
Research has consistently reported that people in various age and schooling levels might hold inadequate scientific conceptions about diverse physical phenomena (e.g., Canpolat, 2006; Griffiths & Preston, 1992; Haidar, 1997; Margel et al., 2008; Mulford & Robinson, 2002; Novick & Nussbaum, 1978; Pozo & Gomez-Crespo, 2005; Valanides, 2000; Williamson et al., 2004; among others). This study provided additional evidence to the existing studies on different concepts (Calik & Ayas, 2005; Gabel et al., 1987; Ginns & Watters, 1995; Haidar, 1997; Valanides, 2000) and found that like other groups of students, preservice teachers also, had some alternative conceptualizations even about such a fundamental chemistry concept "matter". The findings of a number of studies call upon chemistry teacher educators to take these alternative conceptualizations into consideration and design effective instructional sequences to change them into more scientific views. This study was an attempt to respond to this call. In this study, the researchers designed a specific instruction for preservice chemistry teachers (which was a missing aspect in previous research studies) that drew upon the theoretical and empirical evidence from previous studies suggesting integration of multiple representations into instruction.
The results of the study showed that even though the preservice teachers completed introductory level chemistry courses, they experienced some difficulties in classifying particulate representations of matter prior to the specific instruction. Following the instruction, the statistical analysis of the data indicated that the participant preservice chemistry teachers developed more scientific conceptual understandings about the structure of matter and maintained their understandings to a great extent over a 17-month period. On the other hand, as can be seen in Table 2, although not statistically significant, participants' delayed posttest mean scores for each aspect of the structure of matter were slightly less than their posttest mean scores. This is most probably because a few of the participants could not develop well-established understandings of the concept so that they returned to their previous nonscientific conceptions when a certain period of time has passed.
Moreover, based on the evidence from the frequency of scientifically inappropriate classifications, the results of the study indicated that the instruction was quite effective in changing the participants' particulate understandings in terms of the physical states of matter. On the other hand, concerning the higher number of scientifically inappropriate classifications, the instruction appeared not to be as effective in terms of changing the participants' understandings about the physical composition of matter as compared to the extent of change in the participants' understandings of the physical states of matter. This may be due to not giving an explicit attention to this particular aspect during the instruction. Instructional tasks were designed to help preservice teachers to distinguish pure substances from mixtures, but no special attention was given during the instruction to distinguish homogeneous mixtures from heterogeneous mixtures.
Another finding was that the effect of the instruction for overcoming difficulties related to the chemical composition of matter was stronger than the other two aspects, immediately after the instruction. This implied that preservice teachers were better able to identify whether a particulate representation composing of element/s only, compound/s only or both element/s and compound/s after the instruction. However, such change in preservice teachers' understandings of chemical composition of matter was not durable (see Table 8), because 17 months after the instruction, some of the participants who misclassified the given representations with respect to the chemical composition of matter on the pretest reverted back to their initial classifications.
To sum up, based on the analysis of number of scientifically appropriate classification, it can be concluded that the designed instruction was useful for developing the preservice chemistry teachers' understandings of the particulate structure of matter and maintaining such understandings over a 17-month period. On the other hand, based on the analysis of change in scientifically inappropriate classifications for each aspect of the structure of matter, a number of preservice teachers developed more scientific conceptual understandings immediately after the instruction and maintained such understandings over a period of time. However, there were some participants that followed one of the following paths after the instruction: (a) A group of students developed scientific understandings immediately after the instruction but then they reverted back to their initial understandings 17 months after the instruction (e.g., Students 1, 3, 7, 18). This provided further evidence about the robust nature of pre-existing nonscientific conceptions indicated in science education literature. The findings of previous research showed that existing nonscientific conceptions could never be entirely extinguished and then replaced by the scientific ideas; in fact, such ideas usually continue to be maintained in particular contexts (Duit & Treagust, 2003; Novak, 1988; Taber & Tan, 2011). (b) The other group of students changed some of their scientifically inappropriate classifications with the scientific ones, but they also exhibited newly developed ones following the instruction and maintained such scientifically inappropriate classifications over a 17- month period (e.g., Student 3, 4, 9, 11, 13, 16). Perhaps these scientifically inappropriate classifications could be the instruction-induced ones such that students probably misinterpreted the particular concepts during the instruction and generated improper associations among the concepts under investigation in this study (Duit & Treagust, 2003; Mayer, 2009). Thus, such erroneous associations among concepts resulted in the appearance of new nonscientific classifications following the instruction, (c) Another group of students exhibited a kind of scientifically inappropriate classifications only on the delayed posttest but not on the pre and posttest (Students 9, 16, 18, 19). These inappropriate classifications may have to do with the participants' attention or further experiences over a 17-month period in other classes. That is, they might not have paid enough attention while offering response to the questions on the delayed posttest or they had different experiences in other classes over a 17-month period that directed them consider the given representations in a different, scientifically inappropriate, manner.
Implications and Recommendations for Further Research
These findings suggested that science teacher educators should not assume that preservice chemistry teachers possess all the required subject matter knowledge in their subject-related courses. Thus, activities and discussions emphasizing subject matter knowledge about fundamental concepts can be integrated into chemistry teacher education courses (e.g., teaching methods courses, practice teaching courses, etc.). Based on the findings of the present study, science teacher educators might consider using multiple representational tasks combined with discussions and collaborative work to offer preservice chemistry teachers opportunities for learning the particular content. Such opportunities might both help preservice teachers constructing more scientific conceptions and provide them ideas about how to use similar strategies in their future teaching careers. These types of activities might serve pre service teachers to think deeply on fundamental concepts of chemistry and also realize their inadequate and/or nonscientific conceptions.
Apart from recommendations to teacher educators, this study also offers some methodological suggestions to chemistry education researchers. In this study, data analyses were carried out according to both number of scientifically appropriate classifications and the types of inappropriate classifications made by preservice teachers. The results of these two types of analyses were quite parallel to each other and showed that a specific instruction which integrated the use of multiple representations promoted the development of preservice chemistry teachers' scientific understandings of the structure of matter. On the other hand, it should be noted that the effect of the instruction appeared extremely positive based on the results of statistical analyses when only scientifically appropriate classifications were analyzed. When the researchers deeply focused on the participants' scientifically inappropriate classifications of the structure of matter, a little different and more accurate picture regarding the effect of the instruction emerged. The difference between these two types of analyses can be considered as a methodological contribution of the present study. So, instead of using a single lens, the use of complementary analyses could provide more comprehensive information about students learning and consequently about the effectiveness of the instructions.
References
- Adbo, K., & Taber, K. S. (2009). Learners' mental models of the particle nature of matter: A study of 16 year-old Swedish science students. International Journal of Science Education, 31 (6), 757-786.
- Ardac, D., & Akaygun, S. (2004). Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change. Journal of Research in Science Teaching, 41(4), 317-337.
- Ardac, D., & Akaygun, S. (2005). Using static and dynamic visuals to represent chemical change at molecular level. International Journal of Science Education, 27(11), 1269-1298.
- Azizoglu, N., Alkan, M., & Geban, O. (2006). Undergraduate preservice teachers' understandings and misconceptions of phase equilibrium. Journal of Chemical- Education, 83(6), 947-953.
- Baneqee, A. C. (1991). Misconceptions of students and teachers in chemical equilibrium. International Journal of Science Education, 13(4), 487-494.
- Blake, В , Hogue, E., & Sarquis, J. L. (2006). Classifying matter: A physical model using paper dips. Journal of Chemical Education, 83(9), 1317-1318.
- Campbell, D. T., & Stanley, J. C. (1966). Experimental and quasi-experimental designs for research. Chicago: Rand McNally.
- Calik, M., & Ayas, A. (2005). A comparison of level of understanding of eigth-grade students and science student teachers related to selected chemistry concepts. Journal of Research in Science Teaching, 42(6), 638-667.
- Calik, M., Ayas, A., & Coll, R. K. (2007). Enhancing pre-service primary teachers' conceptual understanding of solution chemistry with conceptual change text. International Journal of Science and Mathematics Education, 5(1), 1-28.
- Canpolat, N. (2006). Turkish undergraduates' misconceptions of evaporation, evaporation rate and vapour pressure. International Journal of Science Education, 28(15), 1757-1770.
- Canpolat, N., Pinarbasi, T., & Sozbilir, M. (2006). Prospective teachers' misconceptions of vaporization and vapor pressure. Journal of Chemical Education, 83( 8), 1237-1242.
- Chandrasegaran, A. L., Treagust, D. F., & Mocerino, M. (2007). The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students' ability to describe and explain chemical reactions using multiple levels of representation. Chemistry Education Research and Practice, 8(3), 293-307.
- Chandrasegaran, A. L., Treagust, D. F., & Mocerino, M. (2008). An evaluation of a teaching intervention to promote students' ability to use multiple levels of representation when describing and explaining chemical reactions. Research in Science Education, 38(2). 237-248.
- Cheng, M., & Gilbert, J. K. (2009). Towards a better utilization of diagram in research into the use of representative levels in chemical education. In J. K. Gilbert & D. F. Treagust (Eds), Multiple representations in chemical education, (pp. 55-73). Dordrecht: Springer.
- Driver, R., Squires, A., Rushworth, P., & Wood-Robinson, V. (1994). Making sense of secondary science research into children 's ideas. New York: Routledge.
- Duit, R. (2009). Bibliography: Students' and teachers' conceptions and science education. Retrieved Sept 1, 2011 from, http://www.ipn.uni-kiel.de/aktuell/stcse/stcse .html.
- Duit, R, Treagust, D. F. (1995). Students' conceptions and constructivist teaching approaches. In B. J. Fraser & H. J. Walberg (Eds), Improving Science Education, (pp. 46-49). The Chicago: National Society for the Study of Education.
- Duit, R, & Treagust, D. F. (2003). Conceptual change: A powerful framework for improving science teaching and learning. International Journal of Science Education, 25(6), 671- 688.
- Ebenezer, J. V. (2001). A hypermedia environment to explore and negotiate students' conceptions: Animation of the solution process of table salt. Journal of Science Education and Technology, 10( 1), 73-92.
- Gabel, D. L., Samuel, К. V., & Hunn, D. J. (1987). Understanding the particulate nature of matter. Journal of Chemical Education, 64, 695-697.
- Gabel, D. L. (1993). Use of particle nature of matter in developing conceptual understanding. Journal of Chemical Education, 70(3), 193-194.
- Gabel, D. L. (1998). The complexity of chemistry and implications for teaching. In B. J. Fraser & K. G. Tobin (Eds), International Handbook of Science Education, (pp. 233-248). London, Great Britain: Kluwer Academic Publishers.
- Garnett, P. J., Garnett, P. J., & Hackling, M. W. (1995). Students' alternative conceptions in chemistry: A review of research and implications for teaching and learning. Studies in Science Education, 25, 69-95.
- Gibbons, J. D. (1993). Nonparametric statistics: An introduction. California: Sage Publications.
- Gilbert, J. K., & Treagust, D. F. (2009). Macro, submicro and symbolic representations and the relationships between them: Key models in chemical education. In J. K. Gilbert & D. F. Treagust (Eds), Midtiple representations in chemical education, (pp.1-8). Dordrecht: Springer.
- Ginns, I. S., & Watters, J. J. (1995). An analysis of scientific understandings of preservice elementary teacher education students. Journal of Research in Science Teaching, 32(2), 205-222.
- Griffiths, A. К, & Preston, K. R. (1992). Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules. Journal of Research in Science Teaching, 29(6), 611-628.
- Haidar, A. H. (1997). Prospective chemistry teachers' conceptions of the conservation of matter and related concepts. Journal of Research in Science Teaching, 34(2), 181-197.
- Haidar, A. H, Abraham, M. R. (1991). A comparison of applied and theoretical knowledge of concepts based on particulate nature of matter. Journal of Research in Science Teaching, 28, 919-938.
- Harrison, A. G., & Treagust, D. F. (2002). The particulate nature of matter: Challenges in understanding the submicroscopic world. In J. K. Gilbert, O. De Jong, R. Justi, D. Treagust, & J. H. Van Driel (Eds), Chemical education: Towards research-based practice, (pp. 189-212). Dordrect: Kluwer Academic Publishers.
- Hinton, M. E, & Nakhleh, M. B. (1999). Students' microscopic, macroscopic, and symbolic representations of chemical reactions. The Chemical Educator, 7(5), 158-167.
- Jarvis, T., McKeon, F., & Taylor, N. (2005). Promoting conceptual change in pre-service primary teachers through intensive small group problem-solving activities. Canadian Journal of Science, Mathematics and Technology Education, 5(1), 21-39.
- Johnstone, A. H. (1993). The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70, 701-705.
- Kind, V. (2004). Beyond appearances: Students' misconceptions about basic chemical ideas (2nd Edition). Retrieved March 1, 2012 from, http://www.rsc.org/images/Misconceptions_update_tern18-188603.pdf.
- Kokkotas, P , Vlachos, I, & Koulaidis, V. (1998). Teaching the topic of the particulate nature of matter in prospective teachers' training courses. International Journal of Science Education, 20(3), 291-303.
- Kozma, R. (2003). The material features of multiple representations and their cognitive and social affordances for science understanding. Learning and Instruction, 13(2), 205-226.
- Kozma, R, & Russell, J. (1997). Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. Journal of Research in Science Teaching, 34(9), 949-968.
- Kruse, R. A., & Roehrig, G. H. (2005). A comparison study: Assessing teachers' conceptions with the chemistry concepts inventory. Journal of Chemical Education, 82(8), 1246- 1251.
- Lin, H, Cheng, H, & Lawrenz, F. (2000). The assessment of students and teachers' understanding of gas laws. Journal of Chemical Education, 77(2), 235-238.
- Liu, X, & Lesniak, К. M. (2005). Students' progression of understanding the matter concept from elementary to high school. Science Education, 89(3), 433-450.
- Margel, H , Eylon, В , & Scherz, Z. (2008). A longitudinal study of junior high school students' conceptions of the structure of materials. Journal of Research in Science Teaching, 45(1), 132-152.
- Mayer, R. E. (2003). The promise of multimedia learning: Using the same instructional design methods across different media. Learning and Instruction, 13, 125-139.
- Mayer, R. E. (2009). Multimedia learning (2nd edt.). New York: Cambridge University Press.
- Mulford, D. R, & Robinson, W. R. (2002). An inventory for alternate conceptions among first- semester general chemistry students. Journal of Chemical Education, 79(6), 739-744.
- Novak, J. D. (1988). Learning science and the science of learning. Studies in Science Education, 15, 77-101.
- Novick, S., & Nussbaum, J. (1978). Junior high school pupils' understanding of the particulate nature of matter: An interview study. Science Education, 62, 273-281.
- Nurrenbem, S. C , & Pickering, M. J. (1987). Concept learning versus problem solving: Is there a difference. Journal of Chemical Education, 64(6), 508-510.
- Ozden, M. (2009). Prospective science teachers' conceptions of the solution chemistry. Journal of Baltic Science Education, 8(2), 69-78.
- Pozo, J. I, & Gomez-Crespo, M. A. (2005). The embodied nature of implicit theories: The consistency of ideas about the nature of matter. Cognition and Instruction, 23(3), 351- 387.
- Sanger, M. J. (2000). Using particulate drawings to determine and improve students' conceptions of pure substances and mixtures. Journal of Chemical Education, 77(6), 762-766.
- Shulman, L. S. (1987). Knowledge and teaching: Foundations of new reform. Harvard Educational Review, 57, 1-22.
- Stains, M., & Talanquer, V. (2007a). Classification of chemical substances using particulate representations of matter: An analysis of student thinking. International Journal of Science Education, 29(5), 643-661.
- Stains, M., & Talanquer, V. (2007b). A2: Element or compound? Journal of Chemical Education, 84(5), 880-883.
- Stieff, M., Hegarty, M., & Deslongchamps, G. (2011). Identifying representational competence with multi-representational displays. Cognition and Instruction, 29(1), 123-145.
- Sweller, J., & Chandler, P. (1991). Evidence for cognitive load theory. Cognition and Instruction, 8(4), 351-362.
- Taber, K. S. (2002). Chemical misconceptions: Prevention, diagnosis and cure. Vol: 1. Theoretical background. London: Royal Society of Chemistry.
- Taber, K. S., & Tan, К. C. D. (2011). The insidious nature of‘hard-core' alternative conceptions: Implications for the constructivist research programme of patterns in high school students' and pre-service teachers' thinking about ionisation energy. International Journal of Science Education, 33(2), 259-297.
- Tan, К. C. D., & Taber, K. S. (2009). Ionization energy: Implications of preservice teachers' conceptions. Journal of Chemical Education, 86(5), 623-629.
- Tasker, R., Dalton, R. (2006). Research into practice: Visualization of the molecular world using animations. Chemistry Education Research and Practice, 7(2), 141-159.
- Taylor, N.. & Coll, R. (1997). The use of analogy in the teaching of solubility to preservice primary teachers. Australian Science Teachers ’ Journal, 43(4), 58-64.
- Tsai, C. (1999). Overcoming junior high school students' misconceptions about microscopic views of phase change: A study of an analogy activity. Journal of Science Education and Technology<3(1), 83-91.
- Valanides, N. (2000). Primary student teachers' understanding of the particulate nature of matter and its transformations during dissolving. Chemistry Education: Research and Practice in Europe, 1, 249-262.
- Williamson, V. M., & Abraham, M. R. (1995). The effects of computer animation on the particulate mental models of college chemistry students. Journal of Research in Science Teaching, 32(5), 521-534.
- Williamson, V., Huffman, J., & Peck, L. (2004). Testing students' use of the particulate theory. Journal of Chemical Education, 81(6), 891-896.
- White, R. T., & Arzi, H. J. (2005). Longitudinal studies: Designs, validity, practicality, and value. Research in Science Education, 35(1), 137-149.
- Yezierski, E. J., Birk, J. P. (2006). Misconceptions about the particulate nature of matter: Using animations to close the gender gap. Journal of Chemical Education, 83(6), 954-960.