Personal submersibles offer novel ecological research access to Antarctic waters: an example, with observations of the rarely encountered scyphozoan Stygiomedusa gigantea


Published: Jan. 1, 2023
Latest article update: July 25, 2023
Abstract
Underwater biological surveys have been conducted around the Antarctic continent for several decades, and our knowledge of the species present in the shallow waters (<50 m) is reasonably comprehensive. However, the waters below 50 m remain underexplored on the account of difficulty of access, financial barriers and relatively few operational platforms capable of deployment to such depths. Here, we demonstrate that personal submersibles, now increasingly deployed by the expedition cruise industry, can be vessels of opportunity for biological research in the polar regions. We describe direct observations of the rarely encountered scyphozoan Stygiomedusa gigantea at water depths of 80–280 m in Antarctic Peninsula coastal waters as an example of the potential that personal submersibles present for the scientific community, and we outline possible research avenues for utilizing these platforms in the future.
Keywords
Citizen science, polar, tourism, jellyfish, medusa, Submarine
Introduction
The ecology of the mesopelagic and lower epipelagic zones around Antarctica is relatively understudied, in part because of the logistical and financial implications of operating at such depths. This can also be considered true for the Antarctic benthic environment below scientific SCUBA survey depths, typically around 50 m. The increasing deployment of recreational manned submersibles from expedition cruise vessels in the waters around the Antarctic Peninsula provides a novel opportunity to expand our understanding of the marine ecology of this understudied environment.
Submersibles have long been utilized for biological surveys in depths beyond that accessible by SCUBA (Fricke & Meischner 1985; Lorance et al. 2000; Lindsay & Hunt 2005; Baker et al. 2021). Typically, these submersibles have been owned by nation states and operated by national or academic research institutions (e.g., Grassle et al. 1975; Hissmann & Schauer 2017). However, from the late 1990s and early 2000s, several manufacturers (including U-Boat Worx, Personal Submersibles Organisation, Triton and others) began producing PSs. These PSs have now been deployed extensively throughout the world ocean for recreational (Fairley 2018) and military use, and for commercial purposes by the oil and gas industry (Love & York 2005; Meyer-Gutbrod et al. 2019). PS deployment for scientific use is increasingly common (Schrope 2013; Phillips et al. 2018), although in the polar regions, the majority of deployments have been for film production (Amsler Margaret et al. 2017) or, since 2019, for purely recreational use. The increasing deployment of PSs in the polar regions by the expedition cruise industry represents a novel platform for research when these vehicles are considered as vessels of opportunity. Especially when combined with the advanced research facilities found aboard several new expedition cruise ships, these vessels of opportunity are potentially powerful assets for the scientific community.
The large scyphozoan Stygiomedusa gigantea (Browne, 1910; Cnidaria: Scyphozoa: Semaeostomeae) is a primarily deep-water (1000–3000 m) species, though observed at shallower depths (0–1000 m) in the Southern Ocean. It has been recorded in all ocean basins except the Arctic. Stygiomedusa gigantea is currently the only known species within the genus Stygiomedusa (Matsumoto et al. 2003) since S. stauchi Repelin, 1967 and S. fabulosa Russell, 1959 are now considered synonyms of S. gigantea. The body form of S. gigantea consists of a smooth and rounded bell, curtained with a pleated skirt and four oral arms. Typically, this species appears a deep brown–red hue with a yellow shading to the underside of the bell. Stygiomedusa gigantea individuals can reach substantial sizes, with oral arm lengths of over 6 m and bell diameters of 1 m being not unusual for fully mature specimens (Benfield & Graham 2010). Stygiomedusa gigantea is believed to be viviparous (Russell & Rees 1960).
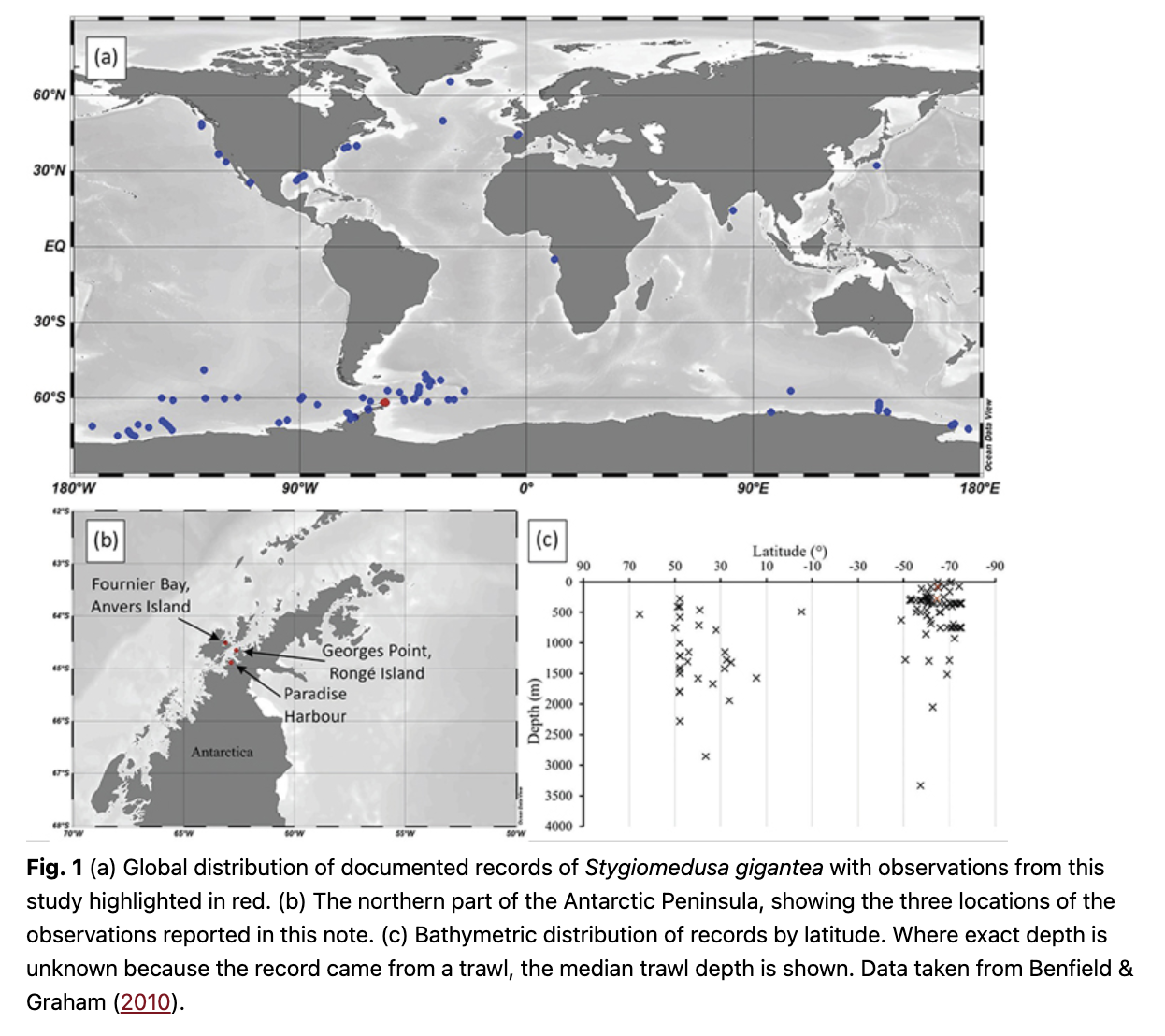
Despite their size, records of S. gigantea remain few, with only 126 recorded encounters (including individuals brought up in nets) since the species was described in 1910 (Benfield & Graham 2010; Tarling et al. 2012; Schiariti et al. 2018; Fig. 1a). The bathymetric distribution of recorded encounters suggests an increased tendency towards shallower depths when in high southern latitudes (Fig. 1c). As a result of the limited number of records, very little is known regarding this species’ full distribution, behaviour, environmental preferences, diet or reproductive cycle. It is therefore important for the scientific community to seize any new opportunity to directly observe S. gigantea in its natural habitat.
Here, we present data from direct observations of S. gigantea conducted from PSs deployed from the expedition cruise vessel Viking Octantis during the 2021/22 Antarctic summer season. Furthermore, we outline the opportunities for utilizing PS as vessels of opportunity for scientific research.
Observations
Twenty-five PS dives were undertaken in a U-Boat Worx Cruise Sub-7 MKII 300 from the expedition cruise vessel Viking Octantis off the Antarctic Peninsula over the period January–March 2022. Dives lasted a maximum of 84 minutes and reached a maximum depth of 300 m. These dives included both training dives and commercial dives with fee-paying guests aboard. During these dives, observations of the large scyphozoan Stygiomedusa gigantea were recorded via mobile telephone cameras and professional cameras. During each observation, the PS was hovered, and all lights remained on.
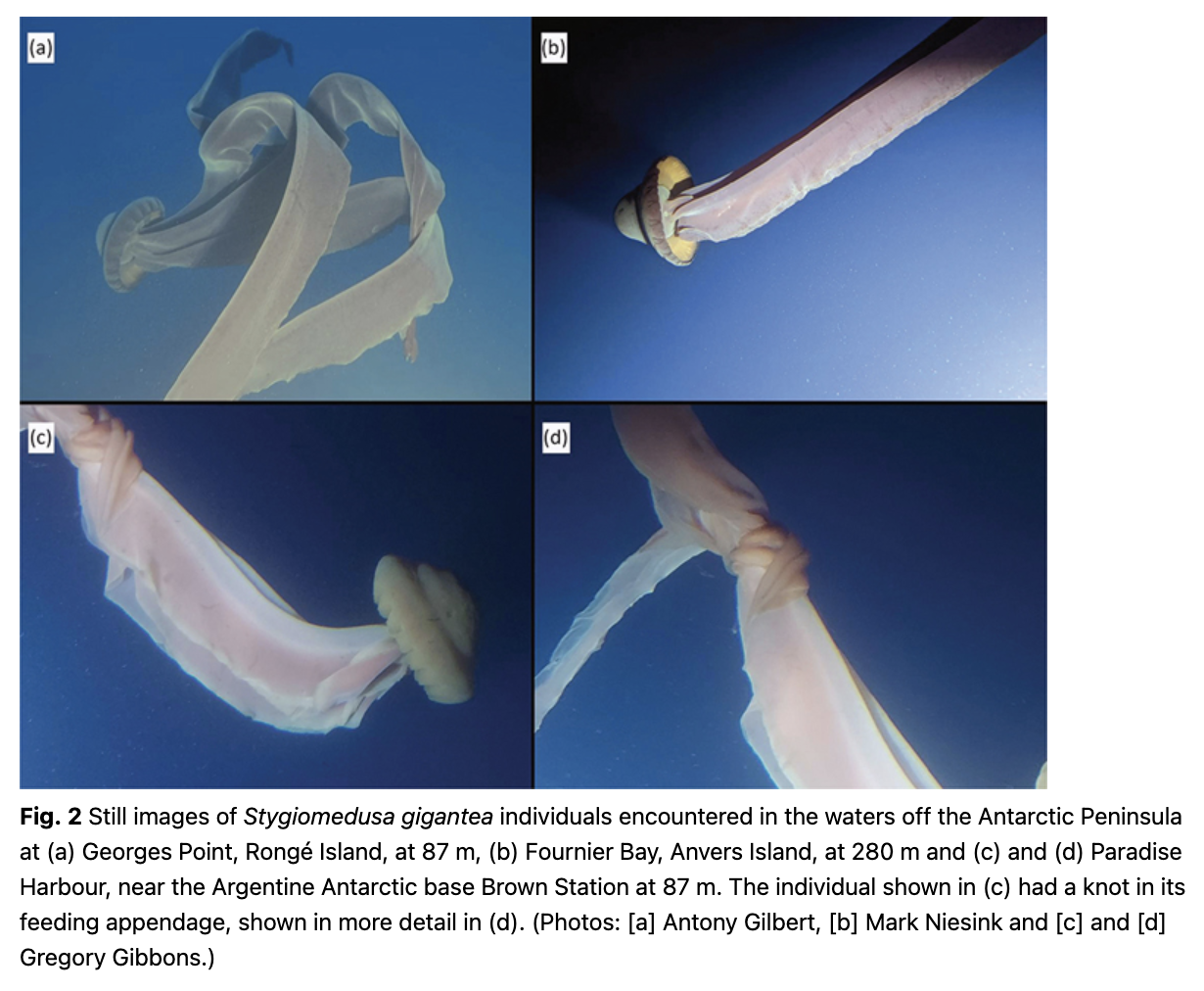
Direct observations of Stygiomedusa gigantea were made from PSs deployed from Viking Octantis three times (see Table 1 and Fig. 1b). Occurrences of S. gigantea were recorded at water depths of 80 m, 87 m and 280 m. On no occasion was more than a single S. gigantea individual observed during a dive. Still images of each individual were recorded (Fig. 2), along with additional video footage (Supplementary material). The three observations were established as different individuals from distinguishing marks on the bell. No quantitative measurements could be made, but each individual observed was estimated to be longer than the length of the PS (4.85 m), and at least double this length for one of the observations. No other species were found in association with S. gigantea individuals. One individual (Fig. 2d) bores a distinct knot in one of its oral arms. Previous observations of S. gigantea made from a PS deployed by the passenger ship the Scenic Eclipse have also been reported from Antarctic Peninsula waters (Fig. 1), although the metadata, including exact location and depth, has been lost (Gilbert, pers. comm. 2022).
Table 1 Dives undertaken with PSs, deployed from Viking Octantis, from which Stygiomedusa gigantea was observed during the 2021/22 Antarctic season. | ||||
Date | Location | Latitude | Longitude | Depth (m) |
20/01/22 | Georges Point, Rongé Island | 64°39’9”S | 62°38’7”W | 80 |
26/01/22 | Fournier Bay, Anvers Island | 64°31’4’’S | 63°8’4’’W | 280 |
15/03/22 | Brown Station, Paradise Harbour | 64°53’9’’S | 62°52’5’’W | 87 |
Discussion
The information in this note highlights the scientific potential of PSs as vessels of opportunity in underwater ecological research. That depths of 50 to 300 m are now being accessed with relative frequency in the polar regions provides novel research opportunity for the scientific community. The multiple observations made of the rarely seen Stygiomedusa gigantea from just a limited number of PS dives provide a clear example of the scientific utility of PSs.
Previous to this publication, there were only 126 documented records (Benfield & Graham 2010; Tarling et al. 2012; Schiariti et al. 2018) of S. gigantea. Of these records, 69% were from the high latitude waters of the Southern Ocean and Antarctica (Benfield & Graham 2010). Previous authors have suggested that the larger proportion of sightings in the Southern Ocean may be due to increased zooplankton sampling effort there. However, the observations presented here add to the emerging impression that the colder waters of the southern high latitudes may be a key habitat for S. gigantea.
All of our observations were made at relatively shallow depths (≤280 m). This is in keeping with an apparent tendency towards shallower depths observed in other recorded encounters made at similar southern latitudes.
A symbiotic relationship has been reported between the ophidiiform fish Thalassobathia pelagica and S. gigantea (Harbison et al. 1973; Drazen & Robison 2004), and it is likely that S. gigantea benefits from the presence of T. pelagica in warmer waters through the removal of parasites (Purcell & Arai 2001). However, T. pelagica is not found in the Southern Ocean, and there are no observations of S. gigantea forming associations with alternative fish species found in the Southern Ocean. We did not observe any other species in association with S. gigantea. It is possible that the lower prevalence and slower growth of parasites in colder waters (Harvell et al. 2002) may mitigate the need for such a symbiosis when S. gigantea is located in southern high latitudes.
Conclusion
Underwater scientific surveys in Antarctica have been conducted via SCUBA since the 1950s (Pollock 2007), with recreational SCUBA operations becoming significant in the early 2000s (Lamers & Gelter 2012). This has led to a fairly good understanding of species found down to 50 m. Below 50 m, the Antarctic marine environment remains under-explored. The increasing deployment of PSs from Antarctic expedition cruise vessels presents a novel opportunity for the scientific community to access these under-explored waters. Additionally, most Antarctic tour operators that deploy PSs also operate Remotely Operated Vehicles for safety reasons, and this presents further opportunities for scientific data collection, as has been demonstrated in other regions of the world (Macreadie et al. 2018; McLean et al. 2020). PSs have the potential to be enhanced for scientific data collection relatively easily by the addition of conductivity–temperature–density sensors (Jonsson et al. 2013), advanced camera arrays (Phillips et al. 2016) and parallel laser systems (Laidig et al. 2013). PS deployment could also be scientifically optimized by dive plans that include same-depth or vertical transects.
Citizen science projects onboard expedition cruise vessels, such as those coordinated by The Polar Collective, are popular with guests and have contributed to numerous scientific publications (e.g., Taylor et al. 2019). There is great potential to harness data from citizen scientists following PS deployment, whereby any georeferenced imagery data are gathered across operators and collectively analysed to build our understanding of polar underwater biotopes and species. Consideration should be given in advance to additional data-gathering equipment required onboard PSs and the storage of data post-dive, including that gathered by citizen scientists.
Acknowledgements
The authors would like to thank all guests and crew of the Viking Octantis, without whom this work would not have been possible. In particular, we thank submarine pilots Mark Andrews, Remy Izendooren and Sven Sudar. We are also grateful to Prof. Julian Dowdeswell and Emily Cunningham for comments that helped to improve this manuscript. Finally, we wish to thank Dr Damon Stanwell-Smith for his scientific oversight at Viking Expeditions, which laid the foundations for this work.
References
| Amsler Margaret O., De Broyer C. & Bolstad K. 2017. In situ submersible observations of western Antarctic Peninsula deep sea fauna. In A. Van de Putte (ed.): Book of abstracts. XIIth SCAR Biology Symposium. Leuven, Belgium, 10–14 July. P. 37. Cambridge: Scientific Committee on Antarctic Research. |
| Baker M.R., Williams K., Greene H.G., Greufe C., Lopes H., Aschoff J. & Towler R. 2021. Use of manned submersible and autonomous stereo-camera array to assess forage fish and associated subtidal habitat. Fisheries Research 243, article no. 106067, doi: 10.1016/j.fishres.2021.106067. |
| Benfield M.C. & Graham W.M. 2010. In situ observations of Stygiomedusa gigantea in the Gulf of Mexico with a review of its global distribution and habitat. Journal of the Marine Biological Association of the United Kingdom 90, 1079–1093, doi: 10.1017/S0025315410000536. |
| Drazen J.C. & Robison B.H. 2004. Direct observations of the association between a deep-sea fish and a giant scyphomedusa. Marine and Freshwater Behaviour and Physiology 37, 209–214, doi: 10.1080/10236240400006190. |
| Fairley P. 2018. Triton submarines’ dive into luxury: these manoeuvrable subs are prized by scientists and yachters alike. IEEE Spectrum 55(10), 17–18, doi: 10.1109/MSPEC.2018.8482415. |
| Fricke H. & Meischner D. 1985. Depth limits of Bermudan scleractinian corals: a submersible survey. Marine Biology 88, 175–187, doi: 10.1007/BF00397165. |
| Grassle J.F., Sanders H.L., Hessler R.R., Rowe G.T. & McLellan T. 1975. Pattern and zonation: a study of the bathyal megafauna using the research submersible Alvin. Deep Sea Research and Oceanographic Abstracts 22, 457–481, doi: 10.1016/0011-7471(75)90020-0. |
| Harbison G.R., Smith K.L. & Backus R.H. 1973. Stygiomedusa fabulosa from the North Atlantic: its taxonomy, with a note on its natural history. Journal of the Marine Biological Association of the United Kingdom 53, 615–617, doi: 10.1017/S0025315400058811. |
| Harvell C.D., Mitchell C.E., Ward J.R., Altizer S., Dobson A.P., Ostfeld R.S. & Samuel M.D. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162, doi: 10.1126/science.1063699. |
| Hissmann K. & Schauer J. 2017. Manned submersible “JAGO”. Journal of Large-Scale Research Facilities 3, article no. A110, doi: 10.17815/jlsrf-3-157. |
| Jonsson J., Smedfors K., Nyholm L. & Thornell G. 2013. Towards chip-based salinity measurements for small submersibles and biologgers. International Journal of Oceanography 2013, article no. 529674, doi: 10.1155/2013/529674. |
| Laidig T.E., Krigsman L.M. & Yoklavich M.M. 2013. Reactions of fishes to two underwater survey tools, a manned submersible and a remotely operated vehicle. Fishery Bulletin 111, 54–68, doi: 10.7755/FB.111.1.5. |
| Lamers M. & Gelter H. 2012. Diversification of Antarctic tourism: the case of a SCUBA diving expedition. Polar Record 48, 280–290, doi: 10.1017/S0032247411000246. |
| Lindsay D.J. & Hunt J.C. 2005. Biodiversity in midwater cnidarians and ctenophores: submersible-based results from deep-water bays in the Japan Sea and north-western Pacific. Journal of the Marine Biological Association of the United Kingdom 85, 503–517, doi: 10.1017/S0025315405011434. |
| Lorance P., Latrouite D. & Séret B. 2000. Observations of chondrichthyan fishes (sharks, rays and chimaeras) in the Bay of Biscay (North-eastern Atlantic) from submersibles. In B. Séret & J.-Y. Sire (eds.): Procedeedings of the 3rd European Elasmobranch Association Meeting, Boulogne-sur-Mer, 1999. Pp. 29–45. Paris: French Ichthyological Society. |
| Love M.S. & York A. 2005. A comparison of the fish assemblages associated with an oil/gas pipeline and adjacent seafloor in the Santa Barbara Channel, southern California Bight. Bulletin of Marine Science 77, 101–118. |
| Macreadie P.I., Mclean D.L., Thomson P.G., Partridge J.C., Jones D.O.B., Gates A.R., Benfield M.C., Collin S.P., Booth D.J., Smith L.L., Techera E., Skropeta D., Horton T., Pattiaratchi C., Bond T. & Fowler A.M. 2018. Eyes in the sea: unlocking the mysteries of the ocean using industrial, remotely operated vehicles (ROVs). Science of the Total Environment 634, 1077–1091, doi: 10.1016/j.scitotenv.2018.04.049. |
| Matsumoto G.I., Raskoff K.A. & Lindsay D.J. 2003. Tiburonia granrojo n. sp., a mesopelagic from the Pacific Ocean representing the type of a new subfamily (class Scyphozoa: order Semaeostomeae: subfamily Tiburoniinae subfam. nov.). Marine Biology 143, 73–77, doi: 10.1007/s00227-003-1047-2. |
| McLean D.L., Parson M.J.G., Gates, A.R., Benfield M.C., Bond T., Booth D.J., Bunce M., Fowler A.M., Harvey E.S., Macreadie P.I., Pattiaratchi C.B., Rouse S., Partridge J.C., Thomson P.G., Todd V.L.G. & Jones D.O.B. 2020. Enhancing the scientific value of industry remotely operated vehicles (ROVs) in our oceans. Frontiers in Marine Science 7, article no. 220, doi: 10.3389/fmars.2020.00220. |
| Meyer-Gutbrod E.L., Love M.S., Claisse J.T., Page H.M., Schroeder D.M. & Miller R.J. 2019. Decommissioning impacts on biotic assemblages associated with shell mounds beneath southern California offshore oil and gas platforms. Bulletin of Marine Science 95, 683–702, doi: 10.5343/bms.2018.0077. |
| Phillips B.T., Becker K.P., Kurumaya S., Galloway K.C., Whittredge G., Vogt D.M., Teeple C.B., Rosen M.H., Pieribone V.A., Gruber D.F. & Wood R.J. 2018. A dexterous, glove-based teleoperable low-power soft robotic arm for delicate deep-sea biological exploration. Scientific Reports 8, article no. 14779, doi: 10.1038/s41598-018-33138-y. |
| Phillips B.T., Gruber D.F., Vasan G., Roman C.N., Pieribone V.A. & Sparks J.S. 2016. Observations of in situ deep-sea marine bioluminescence with a high-speed, high-resolution sCMOS camera. Deep-Sea Research Part I 111, 102–109, doi: 10.1016/j.dsr.2016.02.012. |
| Pollock N.W. 2007. Scientific diving in Antarctica: history and current practice. Diving and Hyperbaric Medicine 37, 204–211. |
| Purcell J.E. & Arai M.N. 2001. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451, 27–44, doi: 10.1023/A:1011883905394. |
| Russell F.S. & Rees W.J. 1960. The viviparous scyphomedusa Stygiomedusa fabulosa Russell. Journal of the Marine Biological Association of the United Kingdom 39, 303–318, doi: 10.1017/S0025315400013345. |
| Schiariti A., Dutto M.S., Pereyra D.Y., Siquier G.F. & Morandini A.C. 2018. Medusae (Scyphozoa and Cubozoa) from southwestern Atlantic and Subantarctic region (32–60 S, 34–70 W): species composition, spatial distribution and life history traits. Latin American Journal of Aquatic Research 46, 240–257, doi: 10.3856/vol46-issue2-fulltext-1. |
| Schrope M. 2013. Giant squid filmed in its natural environment. Nature, doi: 10.1038/nature.2013.12202. |
| Tarling G.A., Stowasser G., Ward P., Poulton A.J., Zhou M., Venables H.J., McGill R.A.R. & Murphy E.J. 2012. Seasonal trophic structure of the Scotia Sea pelagic ecosystem considered through biomass spectra and stable isotope analysis. Deep-Sea Research Part II 59, 222–236, doi: 10.1016/j.dsr2.2011.07.002. |
| Taylor A.R., Barðadóttir Þ., Auffret S., Bombosch A., Cusick A.L., Falk E. & Lynnes A. 2019. Arctic expedition cruise tourism and citizen science: a vision for the future of polar tourism. Journal of Tourism Futures 6, 102–111, doi: 10.1108/JTF-06-2019-0051. |





