Turkish primary students' conceptions about the particulate nature of matter


Published: Jan. 10, 2011
Latest article update: Jan. 4, 2023
Abstract
This study was conducted to determine 4th, 5th, and 6th grade primary students‟ conceptions about the particulate nature of matter in daily-life events. Five questions were asked of students and interviews were used to collect data. The interviews were conducted with 12 students, four students from each grade, after they finished the formal courses related to the particulate nature of matter. The results show that the understanding level of students in all grades about the microscopic properties of matter was quite low. They have little knowledge of or alternative conceptions about the microscopic properties of the particles such as the order of the particles, spaces between particles, the number of particles in different phases, the size of particles and the movement of the particles. And also, progression of students‟ conceptions on the particulate nature of matter is multifaceted. In addition, it was also determined that students have trouble connecting science knowledge to their daily-life experiences.
Keywords
Science Education, primary students, particulate nature of matter, conception
Introduction
Science, especially chemistry, has been regarded as a difficult subject for young students by teachers, researchers and educators. Although the reasons for this vary from the abstract nature of many concepts to the difficulty of the language of science, there are three major reasons for students having difficulties in these areas. One is that topics are very abstract (Ben-Zvi, Eylon, & Silberstein, 1988). Another is that words from everyday language are used but with different meanings (Bergquist & Heikkinen, 1990), and the third is students' lack of formal operational development and poor visualization ability (Gabel, Samuel, & Hunn, 1987). Literature also indicates another source of difficulty. This is that chemistry is described at three levels, only one of which can be readily observed (Johnstone, 1991, Tsaparlis, 1997). These levels are macroscopic, submicroscopic and symbolic and conceptual understanding in chemistry including the ability to represent and translate chemical problems between these levels (Harrison & Treagust, 2000; Johnstone, 1991; 1993; Raviola, 2001). According to Sirhan (2007), the interactions and distinctions between the levels are important characteristics of chemistry learning and necessary for comprehending chemical concepts. Moreover, Hinton and Nakhleh (1999) report that students must use representations characteristic of the macroscopic level, the microscopic level, and the symbolic level for a full understanding chemistry.
The concept of matter is essential to chemistry since chemistry is a science of matter and its transformations. Educators would agree that the particulate nature of matter is part of the heart of theoretical chemistry and the particle theory of matter is a key component in several science education curricula from as early as upper primary school years to various stages of secondary school (Snir, Smith, & Raz, 2003; Treagust et al., 2010). Appropriate understanding of particle theory is essential to learning of chemical concepts (Snir et al., 2003). Although the majority of the students would have heard from different sources the scientifically accepted idea that matter is made of discrete particles that are in constant motion and have empty space between them, they have difficulties applying this concept to actual situations and this causes many problems in the process of learning science (Tsai, 1999). Also, the particulate nature of matter underpins student understanding of many chemistry concepts such as the structure of matter and phase changes (Osborne & Cosgrove, 1983; Bar, 1989; Gabel et al., 1987; de Vos & Verdonk, 1996), diffusion, the dissolution process, solution chemistry (Lee et al., 1993; de Vos & Verdonk, 1996), chemical reactions, and the effect of pressure, volume, and temperature on gases (Nakhleh, 1992).
Because of this importance, there have been made numerous studies related to the particulate nature of matter in the last three decades. In fact, Talanquer (2009) states that the structure of matter and its changes may be one of the topics in which the description of students' ideas at different levels may be best characterized. While some of these studies investigated the student understanding and alternative conceptions (Adbo & Taber, 2009; Ayas & Özmen, 2002; Ayas, Özmen, & £alik, 2010; Boz, 2006; Bouwma-Gearhart, Stewart, & Brown, 2009; de Vos & Verdonk, 1996; Flores-Camacho et al., 2007; Gabel et al., 1987; Garcia Franco & Taber, 2009; Hatzinikita, Koulaidis, & Hatzinikitas, 2005; Jimenez Gomez, Benarroch, & Marin, 2006; Johnson & Papageorgiou, 2010; Liu & Lesniak, 2005; 2006; Lofgren & Hellden, 2008; Margel, Eylon, & Scherz, 2008; Nakhleh, Samarapungavan, & Saglam, 2005; Othman, Treagust, & Chandrasegaran, 2008; Özmen & Kenan, 2007; Papageorgiou & Johnson, 2005; Pozo & Gomez, Crespo, 2005; Talanquer, 2009; Treagust et al., 2010; Wu, Krajcik, & Soloway, 2001; Ydmaz & Alp, 2006), others chose to examine the effect of different teaching approaches on students' learning of particulate nature of matter (Adadan, Irving, & Trundle, 2009; Bunce & Gabel, 2002; Johnson & Papageorgiou, 2010; Kokkotas, Viachos, & Koulaidis, 1998; Meheut, 2004; Noh & Scharmann, 1997; Papageorgiou, Johnson, & Fotiades, 2008; Pierri, Karatrantou, & Panagiotakopoulos, 2008; Snir, Smith & Raz, 2003; Stem, Bamea, & Shauli, 2008; Tsai, 1999). Studies of students' understanding of the particulate nature of matter have indicated that students may have a primitive continuous-matter outlook on the physical world, as opposed to the scientifically-accepted particulate model. These studies revealed students' alternative conceptions concerning the following major aspects of matter concept: composition and structure (Benson, Wittrock & Baur, 1993; Kmel, Glazar, & Watson, 2003; Kmel, Watson, & Glazar, 1998), physical properties and change (Kmel, Watson, & Glazar, 1998; Lee et al., 1993), and chemical properties and change (Boo & Watson, 2001; Johnson, 2000; Solomonidou & Stavridou, 2000). In addition, research findings from the literature indicate that issues such as meaning of the term particles, the nature and characteristics of particles, the nature of space between particles, behavior of particles in different states of matter, the size of molecules, and change in the arrangement of the particles during the phase change and chemical processes are the main problematic ones for the students to understand (Boz & Boz, 2008; de Vos & Verdonk, 1996; Griffiths & Preston, 1992; Harrison & Treagust, 1996; Tsai, 1999; Harrison & Treagust, 2002). Difficulty in applying science knowledge learned in schools to daily-life situations is another problematic issue determined from the studies although this is an important issue in science education (Gallagher, 2000). The results of the studies indicate that students have a tendency to use their perceptions of macroscopic changes of a substance to infer its phase change occurring at
the microscopic level and the presence of the particles in three states of matter is counter-intuitive to their knowledge.
Purpose of the Study
Although the age level at which the particulate nature of matter should be introduced to students is somewhat questionable, if science and technology textbooks are examined, atoms, molecules and the particulate nature of matter are depicted even in primary grades (Gabel, Samuel & Hunn, 1987). It is well-known that the particulate nature of matter is a difficult concept and students have many problems while learning this concept. It also seems difficult for students to apply the particle model consistently across different substances (Nakhleh, Samarapungavan, & Saglam, 2005). Although many international studies about students' understanding of the particulate nature of matter have been carried out by using paper-pencil tests, open-ended questions and/or interviews so far, research related to this concept is very limited in Turkey (Ayas & Özmen, 2002; Ayas, Özmen, & öalik, 2010; Boz, 2006; Özmen, Ayas, & Co§tu, 2002; Özmen & Kenan, 2007; Yilmaz & Alp, 2006) perhaps because science education is a newly developing research area starting from 2000s. Also, although there have been numerous international studies investigating progression in children's understanding of the matter concept from elementary to high school (Holgersson & Lofgren, 2004; Jimenez Gomez, Benarroch, & Marin, 2006; Liu & Lesniak, 2005, 2006; Lofgren & Hellden, 2008; Margel, Eylon, & Scherz, 2008; Nakhleh & Samarapungavan, 1999; Nakhleh et al., 2005), there are only few cross-age studies at the primary level in Turkey (Boz, 2006; Özmen & Kenan, 2007). With this in mind, this study may be a starting point for other researchers to make cross-age studies in Turkey. Such data may give teachers, researchers and educators valuable feedback related to students' background and alternative conceptions as well as the effectiveness of their instruction. Within the domain, the study aims to synthesize students' views about the particulate nature of matter within the context of daily life events.
Methodology
Study Context
The unit of “Matter and Change ” is included in the Science and Technology curriculum between grades 4 and 8 in Turkey (ages 10-14) and the content of the unit changes from grade to grade because the spiral curriculum approach is implemented. In grades 4 and 5, general concepts, such as basic, common and distinctive properties of matter, heat-matter interaction, pure matter, solution and mixture, melting point and freezing point, density, evaporation, condensation and boiling are introduced to students. The content of the grade 6 curriculum consists of building blocks of matter, elements, compounds, molecules, physical and chemical change, states of matter and particulate nature of matter, and the particulate nature of matter and heat. Concepts such as elements and their symbols, compounds and their formulas, structure of atoms, electronic configurations, chemical bonding, periodic system, chemical reactions, acids and bases are introduced in grades 7 and 8. As seen from the content, general properties about matter such as particulate nature of it, states of matter and transformations, etc. are given in grades 4, 5, and 6. For this reason, this study was conducted with these grades of students.
Categories | Criteria for the classification |
Understanding | Responses that included partially and totally correct explanations |
Alternative conception | Responses that included illogical or incorrect student answers which could not be accepted as reliable or not related to scientific knowledge |
No understanding | Repeated the question, contained irrelevant information or an unclear response; responses such as “I do not know” or no response |
Sample
Twelve students from grades 4, 5, and 6 were chosen for this study to interview. Four students from each grade levels, who finished their formal course in school related to the particulate nature of matter, were chosen from the ones who had self-confidence and high ability in self expression from each grade based on their teachers' recommendation.
Instrumentation and Analysis
Semi-structured interviews were used to collect data for in-depth investigation of students' conceptions. During the interviews, students were usually asked about daily life situations. The interviews were individually carried out in a laboratory and lasted about 30-35 minutes. Five main open-ended questions were asked and some new questions were added based on the student answers (Abraham, Williamson, & Westbrook, 1994; Boz, 2006). The questions were related to the effect of heat on the gas particles, condensation and evaporation, and dispersion of liquids in each other and adapted from the literature with minor revisions (e.g., Ayas & Özmen, 2002; Ayas, Özmen & £alik, 2010; Özmen, Ayas & Costu. 2002). The content validity of the questions was achieved by asking experts and science and chemistry teachers to evaluate them. In the data analysis process, students' responses were examined thematically and classified into three categories: understanding, alternative conception, no understanding (Abraham et al., 1994; Ayas & Özmen, 2002; Ayas, Özmen & £alik, 2010; Co§tu, 2008). The categories are described in detail in Table 1.
To assure validity of the classification, the students' responses were classified by two researchers. In each of the five questions, the two researchers agreed in about 90% or more of the classifications. If there was a disagreement in classification, it was discussed in detail and an agreement was reached.
Results and Discussion
The findings of the study are presented below. The following abbreviations were used to identify the sources of the excerpts.
Findings Related to the Effect of Heat on the Gas Particles
The first question of the interview is related to the effect of heat on gas particles, and students were asked to draw a figure and explain their reason for such a drawing.
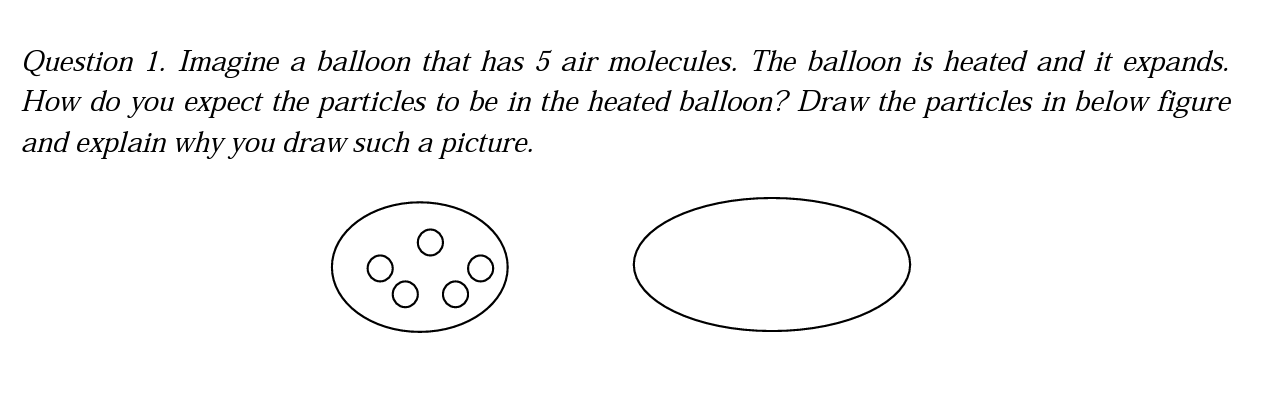
While one of the students from grade 5 gave an explanation in the understanding category, other students' explanations in all grades were placed into the alternative conception category. The student from the grade 5, who gave a correct answer, drew the particles far from each other after the heating and the number of the particles did not change. When he was asked to explain the drawing, he said that the space between the particles in the heated balloon increased, therefore the balloon has expanded. On the other hand, this student drew the particles with a larger size after heating. When asked, he said that the particle size did not change and this was a drawing mistake. Some of the explanations are below:

Students in all grades have two alternative conceptions in common. These are related to particle size and the number of the particles in different phases. Most of the students think that the particle size will increase after the heating and they show such a thought on their drawings. When asked, they also state this view. Most of the studies in international literature report such
an alternative conception (Boz, 2006; Griffiths & Preston, 1992; Lee et al., 1993; Özmen, Ayas & Co§tu, 2002; Özmen & Kenan, 2007; Pereira & Pestana, 1991; Valanides, 2000). For example, Griffiths and Preston (1992) reported similar results that high school students believed that the particle size of a substance would increase as it changes from liquid state to gaseous state or when heated. And also, according to the studies of Özmen, Ayas and Co§tu (2002), Özmen and Kenan (2007) and Pereira and Pestana (1991), most of the students who have this view think that the size of particles will increase during heating and decrease during cooling. On the other hand, Gabel et al. (1987) and Valanides (2000) found that not only students in low grades but also many of the prospective elementary teachers did not conserve the number of the particles and also they believe that the atoms get larger as the matter changes from the liquid to the gas state. This view shows that students attribute the macroscopic properties of matter to its sub- microscopic particles. This is also a commonly reported situation in the literature (Albanese & Vicentini, 1997; Ben-Zvi, Pylon & Silberstein, 1988; Griffiths & Preston, 1992; Harrison & Treagust, 2002; Johnson, 1998; Kokkotas, Vlachos & Koulaidis, 1998; Lee et al., 1993; Nakhleh, 1992). From the students' point of view, it is logical to say that particles expand based on the heat because they know that heated matter expands in daily life. For this reason, it is very common to say that if we heat the particles, they expand.
The other alternative conception was related to the number of the particles and all of the students believed that the number of the particles would increase after heating. When the students were asked how the number of particles increased, one of the grade 4 students based her view on her understanding of evaporation stating that when particles were heated they expand and this causes an increase in the particle number. Another grade 4 student stated that air went into the balloon from outside and this causes an increase in the particle number. Two of the grade 5 students and one of the grade 6 students pointed out that the number of the particles increased by division. The following excerpts illustrate this point:
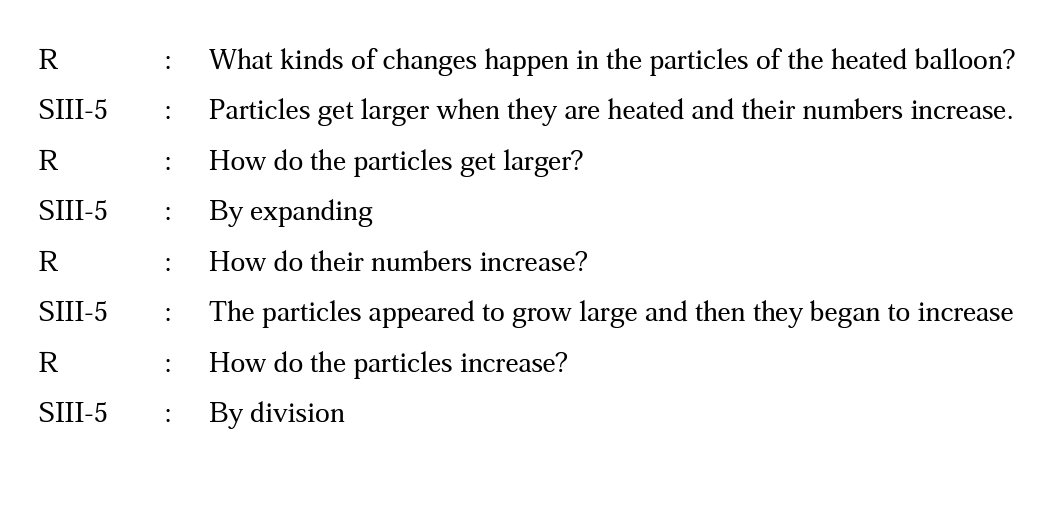
A similar alternative conception is reported in the literature. Studies show that a large number of students hold the beliefs those the atoms get larger as matter changes from the liquid to the gaseous state and the numbers of the particles decrease from the solid to liquid and to gas (Gabel, Hunn & Samuel, 1987; Özmen & Kenan, 2007). Probably, there are two macroscopic views that heated matter gets larger and/or cooled matter gets smaller on the basis of this idea. These results indicate that students have a tendency to use their perceptions on macroscopic changes of a substance to infer its phase change occurring at the microscopic level.
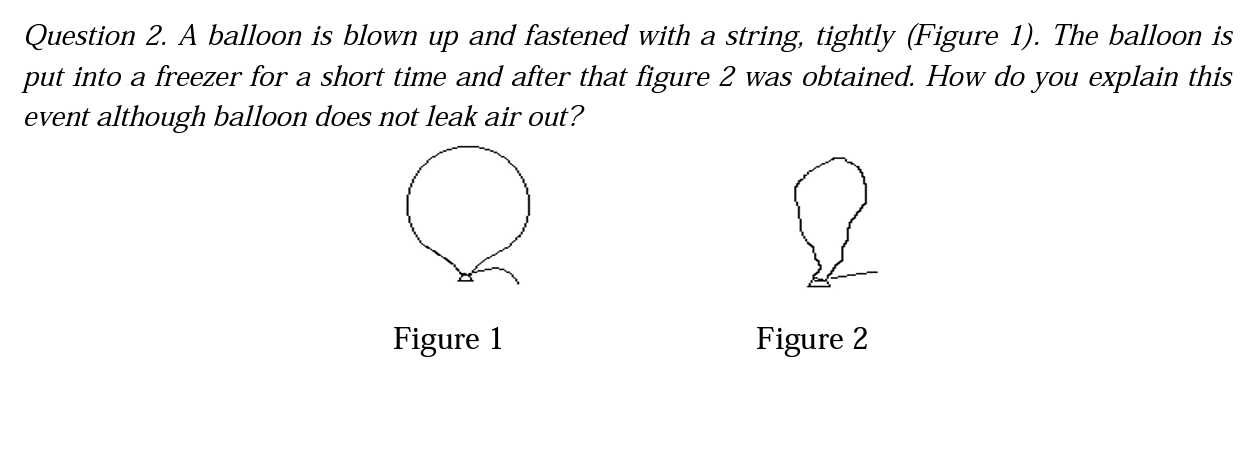
The second question of the interview is also related to the effect of heat on gas particles, and students were asked to explain the phenomena seen in the figures. One of the grade 5 students explained the question in the understanding category while most of them in all grades have the alternative conception. A part of the interview with this student is given in below. Looking at the students' responses, we can understand that this student scientifically explains the effect of cooling on particles.
R | What is the reason for the pucker of the balloon? |
SIV-5 | It expands in hot weather and puckers in cold weather. The volume of the balloon becomes small when it puckers up. Therefore, it becomes a deflated balloon. |
R | What is the thing which puckers in the balloon? |
SIV-5 | The air particles |
R | How do the air particles pucker? |
SIV-5 | Its molecules become closer to one another and the volume of the balloon becomes small. |
Alternative conceptions related to particle size and numbers of the particles are also seen in this question. For example, grade 4 students believed that particles would freeze, they would get smaller, there would be no air in the balloon and the particles would disappear because of cold. Similar alternative conceptions are also reported in related literature (Gabel, Samuel & Hunn, 1987; Griffiths & Preston, 1992; Özmen, Ayas & Co§tu, 2002; Özmen & Kenan, 2007; Pereira & Pest ana, 1991; Valanides, 2000). In these studies, most of the students thought that size of particles decreases during cooling. Some quotations from the students' explain are presented below:

While the grade 5 and grade 6 students have similar alternative conceptions such as the numbers of particles decrease when the balloon is cooled, the particles get smaller when they are cooled and the particles combine with each other and their numbers decrease, one of the grade 6 students pointed out that the reason for the pucker of the balloon is the pressure difference between inside and outside the balloon. This student's explanation is presented below:

Although this student had some ideas related to pressure and behavior of the particles in cold air, he erroneously tried to explain the phenomena air pressure inside and outside the balloon without considering the energy and movement of the particles decreasing inside the balloon.
The findings show that the students had some alternative conceptions about the effects of heat on the gas particles. In general, students think that particle size and number of particles would increase during heating and decrease during cooling. This shows that students do not make the distinction between macroscopic and microscopic properties of matter and attribute the observable changes to microscopic phenomena.
Findings Related to Condensation and Evaporation
The students were asked two questions in order to get their ideas about condensation and evaporation. They were asked the reason of humidity on the bottle taken out from the fridge in one of the questions. This question was related to condensation and the expected reason for this was that the water vapor in the air condensed due to the effect of the cold surface of the bottle.
Question 3. A tightly capped clear bottle filled with a small amount of water is kept in a freezer for a few hours. Many tiny water droplets appeared on the outer surface of the bottle when it is taken out the freezer. How does this phenomenon take place? Please make an explanation.
None of the students could give an acceptable response to this question and most of the responses were placed in alternative conception category. They gave different responses to this question. For example, one student pointed out that the air condensed on the bottle whereas the other one specified that the air included liquid molecules and the humidity resulted from that. The other students think that cold water inside the bottle evaporated when encountering heat. Some student explanations are given below.

When the students' responses are examined, it is seen that none of the students understand the condensation of water vapor found in the air. Actually, we generally say to the students that the air consists of oxygen, nitrogen, carbon dioxide, etc. without mentioning the water vapor. Therefore, students do not think that there is water vapor in air and that it can condense. As a result, they think that air condenses or air turns into water when it comes in contact with a cold surface. Although condensation is a daily-life related concept, a number of studies report alternative conceptions on condensation on cool surfaces (Ayas & Özmen, 2002; Bar & Travis, 1991; Chang, 1999; Co§tu, 2006; Co§tu, 2008; Gopal et al., 2004; Osborne & Cosgrove, 1983; Paik et al., 2004). These studies show that students have several alternative conceptions and difficulties about this topic despite science teachers' extensive efforts in teaching. Literature suggests that connecting science to students' daily-life experiences has been an important issue in science education and this should be included in science lessons (Ogbom et al, 1996). Also, Co§tu (2008) reports that using suitable teaching strategies either helps students in making sense of everyday situations or achieve better conceptual understanding of the concept of condensation. Unfortunately, connecting science knowledge to daily-life events cannot be made effectively in traditional science classrooms and students do not gain better understanding related to these concepts.
The other question which was asked to get students' ideas about evaporation was related to the reason of decreasing water in a glass in front of the window. The expected response for the question is that water particles found in liquid vaporizes when they collect required energy from the environment and other particles. As the glass is open, water vaporizes and run out as time passed.
Question 4. A clear plastic glass filled with a small amount of water is left in front of a window for several days. After the several days, the level gradually diminishes. How do you explain this event by using the particulate nature of matter?
One of the grade 5 and two of the grade 6 students correctly responded to this question. These students called this phenomenon evaporation and explained the reason behind the evaporation correctly. On the other hand, the majority of the students pointed out that it was evaporation and the amount of water in glass decreased because evaporation occurs at all temperatures. Although students knew generally the classical phrase that evaporation occurs at all temperatures, they could not explain this phenomenon at the particulate level. Not using of particle ideas to explain the phenomenon of evaporation in macroscopic terms is also reported in the literature (Özmen, Ayas & Co§tu, 2002; Costu. 2006; Costu. Ayas & Niaz, 2010). Such students' responses were placed in the no understanding category. This is evident from the following interview dialog:

Evaporation may be one of the topics in which the description of students' ideas at different levels may be best characterized (Canpolat, 2006; Chang, 1999; Costu. 2006; Costu & Ayas, 2005; Gopal et al., 2004; Henriques, 2000; Papageorgiou & Johnson, 2005; Prain, Tytler & Peterson, 2009; Tytler, 2000; Tytler & Peterson, 2001, 2004, 2005; Tytler, Prain & Peterson, 2007). In these studies, results show that students at all levels have learning difficulties and alternative conceptions of evaporation and related concepts. The results of this study are parallel to the literature for this topic.
Two of the grade 6 students called the phenomena as evaporation but their responses related to reason were illogical, irrelevant and not acceptable. As follows, one of these students mentioned the formation of rain without giving the expected explanation; the other believed that
hot particles coming from the sun combine the water molecules and water decreases as a result. Two sample dialogs are given below.

In an interesting response, one of the grade 5 students believed that sun light melts the water and water vaporizes. This student is confused in using the words "melt'’ and "vaporize'’. A part of the dialog is presented below.

Generally, the students confused the concepts of evaporation and condensation which they often heard in their daily life. Therefore, they could not explain the situation on the sub- microscopic level, scientifically correct. But, the results of this study show that students are more successful in explaining evaporation related phenomena than the condensation related ones. The reason for this may be that students are more familiar with the evaporation concept than the condensation because they encounter it more in their daily life.
Findings Related to Diffusion of Liquid Particles
The last interview question was related to the dispersion of liquid particles in each other. In this question, the students were asked why ink did not get into the wood when it was dripped on it drop by drop although it got into every part of the water?
Question 5. A big glass is full of water. A few drops of blue ink are dripped into the water. After a while, the color of the water turns into the blue. If the ink is dripped into the wood, does the whole wood turn blue? What is the difference between water and wood?
Two grade 5 and 3 grade 6 students gave explanations which were placed in understanding category. An example of these explanations is given below.

Explanations of one of the grade 6 students showed that this student did not have an adequate understanding about the particulate and spaced nature of the solids. She thought that diffusion of the liquid particles was based on the space between the particles. Some student explanations are given below.
R | Is the ink diffused totally when it is dripped on a piece of wood drop by drop as in water? |
SII-6 | : No, it isn't. |
R | Wiry the ink is not diffused when we drip it onto the piece of wood as in water? |
SII-6 | Because, wood is thick. And also, wood is solid, water is liquid. |
R | Could you please explain your reason in detail? How can you explain this event regarding that matter consists of particles? |
SII-6 | The distance among the particles of water is more than solid ones. Ink spread into the water particles and paints them. On the contrary, there is no space between solid particles. So, ink cannot diffuse between solid particles and cannot paint them. |
One of the grade 4 students stated that wood, like the other solids, did not have particles. This student does not have the scientific knowledge that all matter consists of particles. The explanation of this student is below:
R | Does the ink spread when it is dripped onto a piece of wood drop by drop as in water? |
Sill-4 | No, because it is solid. Water is liquid, wood is solid, so it does not spread |
R | What is the difference between solid and liquid? |
Sill-4 | Liquid always takes the shape of the container in which it founds, but the wood does not. |
R | : How can you explain this event regarding that matters consist of particles? |
Sill-4 | Wood is solid and thick. When you drip the ink, the wood absorbs it. Water is liquid. When it mixes with the ink, it takes a specific color. |
R | : Does the wood have particles? |
Sill-4 | No. Because the wood does not have the particles, the ink does not diffuse in it. |
R | Do not solids have particles? |
Sill-4 | : There is in ice. If you put a glass of water in a fridge, you can see small particles during freezing. But, wood dos not have. |
R | : Does the stone have particles? |
Sill-4 | No, it does not. |
R | Iron? |
Sill-4 | : No. |
This is an interesting and original alternative conception and the reason why students think that solids do not have particles while liquids consist of particles is not clear. Based on students' responses, another alternative conception was determined specifically that ink particles paint the water particles. A part of dialog is an evidence for this alternative conception.

Student responses indicate that students did not have and adequate understanding about diffusion but rather have alternative conceptions such as solids do not have particles, there is no space between solid particles and ink particles paint water particles blue. Some of these alternative conceptions are also reported in the literature (Demircioglu, Akdeniz & Demircioglu, 2004; Griffiths & Preston, 1992; Harrison, 2001; Nakhleh & Samarapungavan, 1999). For example, according to Griffiths and Preston (1992), students believe that particles are in contact and there is no empty space between them. Similarly, Harrison (2001) reported that students view solid particles are in contact, liquid particles about one particle apart and gas particles have 3-4 particles spaces in-between. Also, Pereira and Pestana (1991) found that many high school students have the alternative conception about the relative distance between the particles for the three states. In the literature, Boz (2006) also found that students think that particles in a solid do not have any movement at all because there is no space to go and she explains that this is based on the thought of particles are very close to each other and tightly packed in a solid substance. Based on this explanation, students believe that solid particles are in contact.
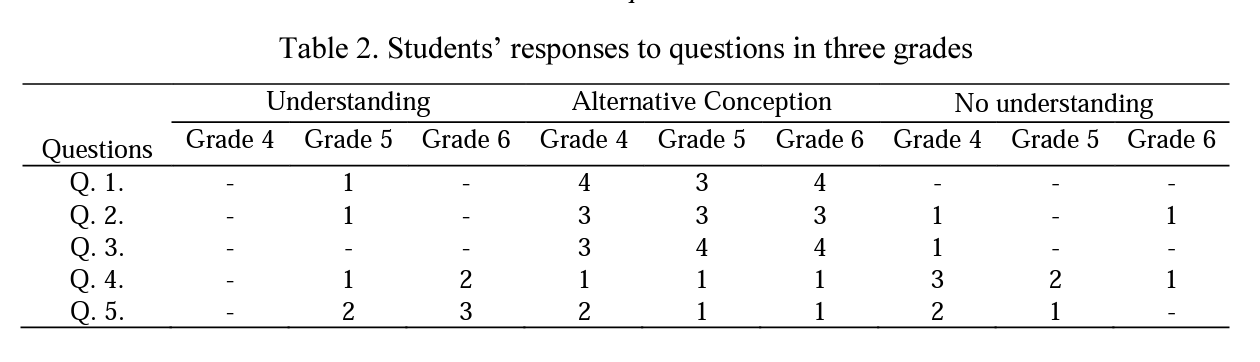
In conclusion, results of this study show that student responses were often categorized in the alternative conception and no understanding categories. Although a few grade 5 and grade 6 student responses to different questions were categorized in the understanding category>, none of the grade 4 students could give responses in this category. Results of the findings are presented in Table 2.
Table 2 means that all of the students in all three grades do not have an adequate understanding of the particulate nature of matter in daily-life events. The first striking thing is that students' understanding levels and alternative conceptions do not change as the grades increase.
Conclusion
Results of this study show that the understanding levels of the students about the microscopic properties of matter are quite low and they have a number of alternative conceptions such as the order, the number, the size, the movement of the particles and spaces between them. Similar alternative views are reported in literature (Boz, 2006; Özmen, Ayas & Co§tu, 2002; Özmen & Kenan, 2007; Valanides, 2000). Two other problematic issues are evaporation and condensation and literature has also several alternative conceptions related to these concepts (Canpolat, 2006; Chang, 1999; Co§tu & Ayas, 2005; Papageorgiou & Johnson, 2005; Prain, Tytler & Peterson, 2009; Tytler & Peterson, 2001; Tytler, Prain & Peterson, 2007). Several studies in the literature on the particulate nature of matter report insufficient understanding of students at all levels (Ayas, Özmen & Qahk, 2010; Boz, 2006; Bouwma-Gearhart, Stewart & Brown, 2009; Hatzinikita, Koulaidis & Hatzinikitas, 2005; Liu & Lesniak, 2005; Lofgren & Hellden, 2008; Nakhleh et al., 2005; Othman, Treagust & Chandrasegaran, 2008). These results show that at least some of the students at every grade fail to understand conceptually the particulate nature of matter even following formal science instruction. Although students are generally familiar with atoms, molecules and generally particles concepts from both popular media and schools and they know that matter is made up of discrete particles, they are reluctant to use the particulate nature of matter to explain observable phenomena and attribute the macroscopic properties of matter to the microscopic level. This has also been documented by previous studies (Abraham et al., 1994; Albanese & Vicentini, 1997; Gabel et al., 1987; Griffiths & Preston, 1992; Harrison & Treagust, 2002; Johnson, 1998; Kokkotas, Vlachos & Koulaidis, 1998). According to Adadan, Irving and Trundle (2009), this may be because the particulate nature of matter competes with what students observe in daily-life. For example, students have difficulty in accepting the notion that a drop of water consists of a large number of particles, that they are in constant motion, and they are attached to each other because they perceive a piece of water as smooth and continuous (Pozo & Gomez Crespo, 2005; Snir, Smith & Raz, 2003).
Another conclusion of this study is that students of all grades in this study are reluctant to use the particulate theory to explain daily-life events. Even when they had some correct ideas, they have difficulties in transferring their theoretical knowledge about the particulate nature of matter to explain daily-life events. Collected data shows that even though most of the students had the classical knowledge that all matter is made of particles; they failed to associate this scientific idea with daily-life events and to use it for explaining observed phenomena. Similar results were obtained from the studies of Ayas and Özmen (2002), Briggs, Brook and Driver (1984), Lofgren and Hellden (2009) and Özmen, Ayas and Costu (2002). For example, Lofgren and Hellden (2009) investigated how students use a molecule concept when explaining everyday situations in a longitudinal study and they found that most students did not connect the knowledge they gain in school about the particulate nature of matter to these everyday situations. This shows that students' knowledge related to particulate theory is simple and dysfunctional. Although connecting science to students' daily-life experiences has been an important issue in science education (Ogbom et al., 1996) and daily-life experiences are a way to make science meaningful to students (Campbell & Lubben, 2000), most of students could not apply their science knowledge learned in schools to daily-life events (Gallagher, 2000). According to Harlen (2002), daily-life theme related to science is necessary to educate students as scientifically literate citizens, but they do not have opportunity to do so in schools (Gallagher, 2000). Based on this, students cannot make the sicence concepts related to daily-life themes meaningful in their minds. This causes the insufficient construction of the scientific knowledge.
All of these results indicate that students' knowledge related to the particulate nature of matter remains as discrete pieces of information in their mind resulting in rote learning. Boz (2006) suggests that teachers in science classrooms should encourage the students to use the particulate nature of matter in explaning daily-life related events to prevent rote learning and to facilitate conceptual understanding of the particle theory.
Interpretation Differences Between Grades
In this study, findings from three grades show that the students are not at the desired level. The majority of the students exhibited very limited understanding of the concept and had difficulties to relate the observable macroscopic changes to the invisible molecular events. They did not develop an understanding on how macroscopic observations might be related to microscopic explanations. It is expected that the levels of conceptions of 4th, 5th and 6th grade students have to increase gradually. Because the concepts related to the particulate nature of matter are taken up more detail with increasing grade, students are expected to explain some daily life events more correctly and successfully in higher grades. The results show that there is not a great conceptual understanding difference between the grades in favor of higher level and progression of students' conceptions on the particulate nature of matter is multifaceted. For example, while grade 4 students did not give responses in the understanding category, grade 5 and grade 6 students' responses were close to each other. On the other hand, it is interesting that in all grades, students' responses in the alternative conception category were similar to each other. This means that although students at each level take several science classes during their schooling, they have alternative conceptions even after learning the correct concepts in the classrooms. In fact, in question 3, it was seen that the alternative conceptions of the students increased as long as their grades increased. This may be because the students want to use the newly learned knowledge without assimilating it completely for explaining events.
In the literature, Liu and Lesniak (2006) state that there is no clear conceptual leap between different grade levels in conceptual progression, that is, there is tremendous overlap in
conceptions among students of different grades. And also, according to Liu and Lesniak (2005), matter concept development in children from elementary to high school undergoes five overlapping waves. The first wave involves developing informal ideas on matter such as properties and changes involving water and air and may occur by grade 3 or 4. The second wave occurs by grade 7 when students develop understanding of the aspect on matter conservation. The third wave is indicated by understanding physical and chemical properties and changes by grade 8 and 12 general students. The fourth wave involves structural and composition aspect of the matter. And the last wave involves explaining and predicting matter and changes using bonding theories. Treagust, Chittieborough and Mamiala (2003) state that only at the last level are students fluent in representing and coordinating matter and changes at the macroscopic, symbolic, and microscopic levels. For this reason, it may be unreasonable to expect conceptual leap between the 4th, 5th, and 6th grade in this study. Gabel, Samuel and Hunn (1987) state that alternative conceptions and lack of understanding of the particulate nature of matter on the part of chemistry students may be related to their lack of formal operational development or to their poor visualization ability. They also think that it is more likely due to their lack of differentiation of concepts such as solids, liquids, gases elements, compounds, substances, mixtures, solutions, and to the lack of instruction in which these terms are related to the particulate nature of matter.
Implications for Teaching
It is also known that at the present time most chemistry courses are taught at the symbolic level with little emphasis on the microscopic and the macroscopic levels and insufficient connections are made between the three levels and the information remains compartmentalized in the longterm memories of students (Gabel, 1993). This causes insufficient understanding of concepts by the students. Therefore, teachers need to emphasize the transitions between the symbolic, macroscopic, and microscopic world so that students will develop their own mental models of the particulate nature of matter on these three levels. This may be only possible by using different ways of teaching of the particulate nature of matter. Computer animations and computerized models may be effective tools to teach the particulate nature of matter because these help students in forming the changes which occur in microscopic level in their minds. Literature also suggests that particle level animations not only help to reconstruct students' primitive mental particle models, but also provided an explanation for macroscopic behavior (Yezierski, 2003). In addition, Tsai (1999) suggests a role-playing and student-centered analogy activity for meaningful learning.
Although the majority of the students have heard from different sources the scientifically accepted idea that matter is made of discrete particles that are in constant motion and have empty space between them, it is known that they have difficulties applying this concept to actual situations and this causes many problems in the process of learning science and therefore creates alternative conceptions (Tsai, 1999). Because the alternative conceptions appear to be resistant to attempts to change them over time despite increased science education, students pass from grade to grade without fully grasping the underlying concepts. For this reason, teachers should also be equipped with the necessary capabilities of continuously identifying their own students' conceptions and implementing teaching approaches that promote conceptual understanding among their students. On the other hand, studies related to alternative conceptions have shown that isolating school science from students' daily-life could make students develop two unconnected knowledge systems related to science; one is used to solve science problems in schools, and the other is used for their daily-lives (Osborn & Freyberg, 1985). Therefore, teachers should consider important students' applying scientific knowledge learned in schools to daily-life
events, which has been accepted an important issue in science education and in making science meaningful to students.
References
- Abraham, M. R., Williamson, V. M., & Westbrook, S. L. (1994). A cross-age study of the understanding of five chemistry concepts. Journal of Research in Science Teaching, 31(2), 147-165.
- Adadan, E., Irving, К. E., & Trundle, К. C. (2009). Impacts of multi-representational instruction on high school students' conceptual understandings of the particulate nature of matter. International Journal of Science Education, 31(13), 1743-1775.
- Adbo, K., & Taber, K. S. (2009). Learners' mental models of the particle nature of matter: A study of 16-year-ols Swedish science students. International Journal of Science Education, 31(6), 757- 786.
- Albanese, A., & Vicentini, M. (1997). Wiry do we believe that an atom is colourless? Reflections about the teaching of the particle model. Science & Education, 6,251-261.
- Ayas, A., & Özmen, H. (2002). A study of students' level of understanding of the particulate nature of matter at secondary school level, Bogazici University Journal of Education, 19(2), 45-60.
- Ayas, A., Özmen, H., & Calik. M. (2010). Students' conceptions of the particulate nature of matter at secondary and tertiary level. International Journal of Science and Mathematics Education, 8, 165-184."
- Bar, V. (1989). Children's views about the water cycle. Science Education, 73, 481-500.
- Bar, V,:, & Travis, A. S. (1991). Children's views concerning phase changes. Journal of Research in Science Teaching, 28, 363-382.
- Benson, D. L., Wittrock, M. C., & Baur, M. E. (1993). Students' preconceptions of the nature of gases. Journal of Research in Science Teaching, 30(6), 587-597.
- Ben-Zvi, R., Eylon, B., & Silberstein, J. (1988). Theories, principles and laws, Education in Chemishy, 25, 89-92.
- Bergquist, W., & Heikkinen, H. (1990). Student ideas regarding chemical equilibrium, Journal of Chemical Education, 66(12), 1000-1003.
- Boo, H. K., & Watson, J. R. (2001). Progression in high school students' (aged 16-18) conceptualizations about chemical reactions in solution. Science Education, 85, 568-585.
- Bouwma-Gearhart, J., Stewart, J., & Brown, K. (2009). Student misapplication of a gas-like model to explain particle movement in heated solids: Implications for curriculum and instruction towards students' creation and revision of accurate explanatory models. International Journal of Science Education, 31(9), 1157-1174.
- Boz, N., & Boz, Y. (2008). A (Qualitative case study of prospective chemistry teachers' knowledge about instructional strategies: Introducing particulate theory. Journal of Science Teacher Education, 19, 135-156.
- Boz, Y. (2006). Turkish pupils' conception of the particulate nature of matter, Journal of Science Education and Technology, 15(2), 203-213.
- Briggs, H., Brook, A., & Driver, R. (1984). Aspects of secondary students' understanding of the particulate nature of matter. CLISP: The University of Leeds.
- Bunce, D. M., & Gabel, D. (2002). Differential effects on the achievement of males and females of teaching the particulate nature of chemistry. Journal of Research in Science Teaching, 39( 10). 911-927.
- Campbell, B., & Lubben, F. (2000). Learning science through context: Helping pupils make sense of everyday situations. International Journal of Science Education, 22(3), 239-252.
- Canpolat, N. (2006). Turkish undergraduates' misconceptions of evaporation, evaporation rate, and vapour pressure. International Journal of Science Education, 28, 1757-1770.
- Chang, J. Y. (1999). Teacher college students' conceptions about evaporation, condensation, and boiling. Science Education, 83, 511-526.
- Costu. B. (2006). Determining students' conceptual change levels: Evaporation, condensation, and boiling. Doctoral Dissertation, Karadeniz Technical University, Trabzon, Turkey.
- Co§tu, B. (2008). Learning science through the PDEODE teaching strategy: Helping students make sense of everyday situations. Eurasia Journal of Mathematics, Science & Technology Education, 4(1), 3-9.
- Co§tu, B., & Ayas, A. (2005). Evaporation in different liquids: Secondary students' conceptions. Research in Science and Technology Education, 23, 73-95.
- Co§tu, B., Ayas, A., & Niaz, M. (2010). Promoting conceptual change in first year students' understanding of evaporation. Chemistry Education: Research and Practice, 11, 5-16.
- de Vos, W., & Verdonk, A. H. (1996). The particulate nature of matter in science education and in science. Journal of Research in Science Education, 33(6), 657-664.
- Demircioglu, H., Akdeniz, A. R., & Demircioglu, G. (2004). Maddenin tanecikli yapisina Hi fan kavram yamlgilanmn giderilmesinde qahpna yapraklanmn etkisi. Paper presented at the XII. annual meeting of Educational Sciences, Gazi University, 15-18 October 2004, Volume III, pp. 2137-2160, Ankara.
- Flores-Camacho, F., Gallegos-Cazares, L., Garritz, A., & Garcia-Franco, A. (2007). Incommensurability and multiple models: Representations of the structure of matter in undergraduate chemistry students. Science & Education, 16, 775-800.
- Gabel, D. L. (1993). Use of the particle nature of matter in developing conceptual understanding, Journal of Chemical Education, 60(3), 193-194.
- Gabel, D. L., Samuel, К. V., & Hunn, D. (1987). Understanding the particulate nature of matter, Journal of Chemical Education, 6-/(8), 695-697.
- Gallagher, J. J. (2000). Teaching for understanding and applications of science knowledge. School Science and Mathematics, 100(6), 310-318.
- Garcia Franco, A., & Taber, K. S. (2009). Secondary students' thinking about familiar phenomena: Learners' explanations from a curriculum context where "particles” is a key idea for organizing teaching and learning. International Journal of Science Education, 31(14), 1917-1952.
- Gopal, H., Kleinsmidt, J., Case, J., & Musonge, P. (2004). An investigation of tertiary students“ understanding of evaporation, condensation and vapor pressure. International Journal of Science Education, 26, 1597-1620.
- Griffiths, A. K., & Preston, K. R. (1992). Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules. Journal of Research in Science Teaching, 29(6), 611- 628.
- Harten, W. (2002). Links to everyday life: The roots of scientific literacy. Primary Science Review, 71, 8-10.
- Harrison, A. G. (2001). Textbooks for outcomes science: A review. The Queensland Science Teacher, 27(6), 20-22.
- Harrison, A. G., & Treagust, D. F. (1996). Secondary students' mental models of atoms and molecules: Implications for teaching chemistry. Science Education, 80, 509-534.
- Harrison, A. G., & Treagust, D. F. (2000). Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in grade 11 chemistry. Science Education, 84, 352-381.
- Harrison, A. G., & Treagust, D. F. (2002). The particulate nature of matter: Challenges in understanding the microscopic world. In J. K. Gilbert et al. (Eds.), Chemical Education: Towards Research-Based Practice, (pp. 189-212). Dordrecht: Kluwer Academic.
- Hatzinikita, V., Koulaidis, V., & Hatzinikitas, A. (2005). Modeling pupils' understanding and explanations concerning changes in matter. Research in Science Education, 35, 471-495.
- Henriques, L. (2000). Children 's misconceptions about weather: A review of the literature, paper presented at the annual meeting of the National Association of Research in Science Teaching, New Orleans, LA.
- Hinton, M. E., & Nahkleh, M. B. (1999). Students“ microscopic, macroscopic, and symbolic representations of chemical reactions. Chemical Educator, 4(4), 1-29.
- Holgersson, I., & Lofgren, L. (2004). A long-term study of students' explanations of transformations of matter. Canadian Journal of Science, Mathematics and Technology Education, 4(1), 77-96.
- Jimenez Gomez, E. J., Benarroch, A., & Marin, N. (2006). Evaluation of the degree of coherence found in students' conceptions concerning the particulate nature of matter. Journal of Research in Science Teaching, 43(6), 577-598.
- Johnson, P. (1998). Children's understanding of changes of state involving the gas state, Part 1: Boiling water and the particle theory. International Journal of Science Education, 20(5), 567- 583.
- Johnson, P. (2000). Children's understanding of substances: Part I. Recognizing chemical change. International Journal of Science Education, 22, 719-737.
- Johnson, P., & Papageorgiou, G. (2010). Rethinking the introductory of particle theory: A substancebased framework. Journal of Research in Science Teaching, 47(2), 130-150.
- Johnstone, A. H. (1991). Wiry is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Learning, 7, 75-83.
- Johnstone, A. H. (1993). The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70, 701-704.
- Kokkotas, P., Vlachos, I., & Koulaidis, V. (1998). Teaching the topic of the particulate nature of matter in prospective teachers training courses. International Journal of Science Education, 20(3), 291-303.
- Kmel, D., Glazar, S. S., & Watson, R. (2003). The development of the concept of "matter": A crossage study of how children classify materials. Science Education, 87(5), 621-639.
- Kmel, D., Watson, R., & Glazar, S. A. (1998). Survey of research related to the development of the concept of "matter”. International Journal of Science Education, 20(3), 257-289.
- Lee, O., Eichinger, D. C., Anderson, C. W., Berkheimer, G. D., & Blakeslee, T. D. (1993). Changing middle school students' conceptions of matter and molecules. Journal of Research in Science Teaching, 30(3), 249-270.
- Liu, X., & Lesniak, K. (2005). Students’ progression of understanding the matter concept from elementary to high school. Science Education, 89, 433-450.
- Liu, X., & Lesniak, K. (2006). Progression in children 's understanding of the matter concept from elementary to high school. Journal of Research in Science Teaching, 43(3), 320-346.
- Lofgren, L., & Hellden, G. (2008). Following young students' understanding of three phenomena in which transformations of matter occur. International Journal of Science and Mathematics Education, 6, 481-504.
- Lofgren, L., & Hellden, G. (2009). A longitudinal study showing how students use a molecule concept when explaining everyday situations. International Journal of Science Education, 31(4), 1631-1655.
- Margel, H., Eylon, B. S., & Scherz, Z. (2008). A longitudinal study of junior high school students' conceptions of the structure of materials. Journal of Research in Science Teaching, 45(1), 132- 152.
- Meheut, M. (2004). Designing and validating two teaching-learning sequences about particle models. International Journal of Science Education, 26(5), 605-618.
- Nakhleh, M. B. & Samarapungavan, A. (1999). Elementary school children's beliefs about matter. Journal of Research in Science Teaching, 36(6), 666-805.
- Nakhleh, M. B. (1992). Wiry some students don't learn chemistry: Chemical misconceptions. Journal of Chemical Education, 69, 191-196.
- Nakhleh, M. B., Samarapungavan, A., & Saglam, Y. (2005). Middle school students’ beliefs about matter. Journal of Research in Science Teaching, 42(5), 581-612.
- Noh, T., & Scharmann, L. (1997). Instructional influence of a molecular-level pictorial presentation of matter on students' conceptions and problem-solving ability. Journal of Research in Science Teaching, 34(2), 199-217.
- Ogbom, J., Kress, G., Martins, I., & McGillicuddy, K. (1996). Explaining science in the classroom. Buckingham: Open University Press.
- Osborne, R. J., & Cosgrove, M. M. (1983). Children's conceptions of the changes of the state of water. Journal of Research in Science Teaching, 20, 825-838.
- Osborne, R. J., & Freyberg, P. (1985). Learning in science: The implications of children 's science. London: Heinemann.
- Othman, J., Treagust, D. F., & Chandrasegaran, A. L. (2008). An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding. International Journal of Science Education, 30(11), 1531-1550.
- Özmen, H., & Kenan, O. (2007). Determination of the Turkish primary students' views about the particulate nature of matter. Asia Pacific Forum on Science Learning and Teaching, 8(1), 1-7.
- Özmen, H., Ayas, A., & Costu. B. (2002). Determination of the science student teachers' understanding level and misunderstandings about the particulate nature of the matter. Educational Sciences: Theory & Practice, 2(2), 506-529.
- Paik, S. H., Kim, H. N., Cho, В. K., & Park, J. W. (2004). K-8th grade Korean students' conceptions of "changes of state" and "conditions for changes of state ”. International Journal of Science Education, 26(2), 207-224.
- Papageorgiou, G., & Johnson, P. (2005). Do particle ideas help or hinder pupils' understanding of phenomena? International Journal of Science Education, 27(11), 1299-1317.
- Papageorgiou, G., Johnson, P., & Fotiades, F. (2008). Explaining melting and evaporation below boiling point. Can software help with particle ideas? Research in Science & Technological Education, 26(2), 165-183.
- Pereira, M. P., & Pestana, M. E. M. (1991). Pupils' representations of water. International Journal of Science Education, 13, 313-319.
- Pierri, E., Karatrantou, A., & Panagiotakopoulos, C. (2008). Exploring the phenomenon of "change of phase" of pure substances using the microcomputer-based-laboratory (MBL) system. Chemistry Education: Research and Practice, 9, 234-239.
- PozOj J. I., & Gomez Crespo, M. A. (2005). The embodied nature of implicit theories: The consistency of ideas about the nature of matter. Cognition and Instruction, 23(3), 351-387.
- Prain, V., Tytler, R., & Peterson, S. (2009). Multiple representation in learning about evaporation. International Journal of Science Education, 31, 787-808.
- Raviola, A. (2001). Assessing students' conceptual understanding of solubility equilibrium. Journal of Chemical Education, 78(5), 629-631.
- Sirhan, G. (2007). Learning difficulties in chemistry: An overview. Journal of Turkish Science Education, 4(2), 2 - 20.
- Snir, J., Smith, C. L., & Raz, G. (2003). Linking phenomena with competing underlying models: A software tool for introducing students to the particulate model of matter. Science Education, 86, 694-830.
- Solomonidou, C., & Stavridou, H. (2000). From inert object to chemical substances: Students initial conceptions and conceptual development during an introductory experimental chemistry sequence. Science Education, 84, 382-400.
- Stem, L., Bamea, N., & Shauli, S. (2008). The effect of a computerized simulation on middle school students' understanding of the kinetic molecular theory. Journal of Science Education and Technology, 17, 305-315.
- Talanquer, V. (2009). On cognitive constraints and learning progression: The case of "structure of matter". International Journal of Science Education, 31(15), 2123-2136.
- Treagust, D. F., Chandrasegaran, A. L., Crowley, J., Yung, В. H. W., Cheong, I. P.-A., & Othman, J. (2010). Evaluating students' understanding of kinetic particle theory concepts relating to the states of matter, changes of state and diffusion: A cross-national study. International Journal of Science and Mathematics Education, 8, 141-164.
- Treagust, D.F., Chittieborough, G., & Mamiala, T.L. (2003). The role of submicroscopic and symbolic representations in chemical explanations. International Journal of Science Education, 25, 1353-1368.
- Tsai, C. -C. (1999). Overcoming junior high school students' misconceptions about the microscopic views of phase change: A study of an analogy activity. Journal of Science Education and Technology, 8(1), 83-91.
- Tsaparlis, G. (1997). Atomic and molecular structure in chemical education: A critical analysis from various perspectives of science education. Journal of Chemical Education, 74, 922-925.
- Tytler, R. (2000). Comparison of year 1 and year 6 students' conceptions of evaporation and condensation: Dimensions of conceptual progression. International Journal of Science Education, 22, 447-467.
- Tytler, R., & Peterson, S. (2001). Deconstructing learning in science: Young children's responses to a classroom sequence on evaporation. Research in Science Education, 30, 339-355.
- Tytler, R., & Peterson, S. (2004). Young children learning about evaporation: Insights from a longitudinal study. Canadian Journal of Science, Mathematics and Technology Education, 4, 111-126.
- Tytler, R., & Peterson, S. (2005). A longitudinal study of children's developing knowledge and reasoning in science. Research in Science Education, 35, 63-98.
- Tytler, R., Prain, V., & Peterson, S. (2007). Representational issues in students' learning about evaporation. Research in Science Education, 37, 313-331.
- Valanides, N. (2000). Primary student teachers' understanding of the particulate nature of matter and its transformations during dissolving. Chemistry Education: Research and Practice in Europe, 1(2), 249-262.
- Wu, H., Krajcik, J. S., & Soloway, E. (2001). Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom. Journal of Research in Science Teaching, 38,821-842.
- Yezierski, E. J. (2003). The particulate nature of matter and conceptual change: A cross-age study. Doctoral Dissertation, Arizona State University, USA.
- Yilmaz, A., & Alp, E. (2006). Students ’ understanding of matter: the effect of reasoning ability and grade level. Chemistry Education Research and Practice, 6, 22-31.





